Live Class - O'level/IGCSE/GCSE Chemistry - Metals Part 2
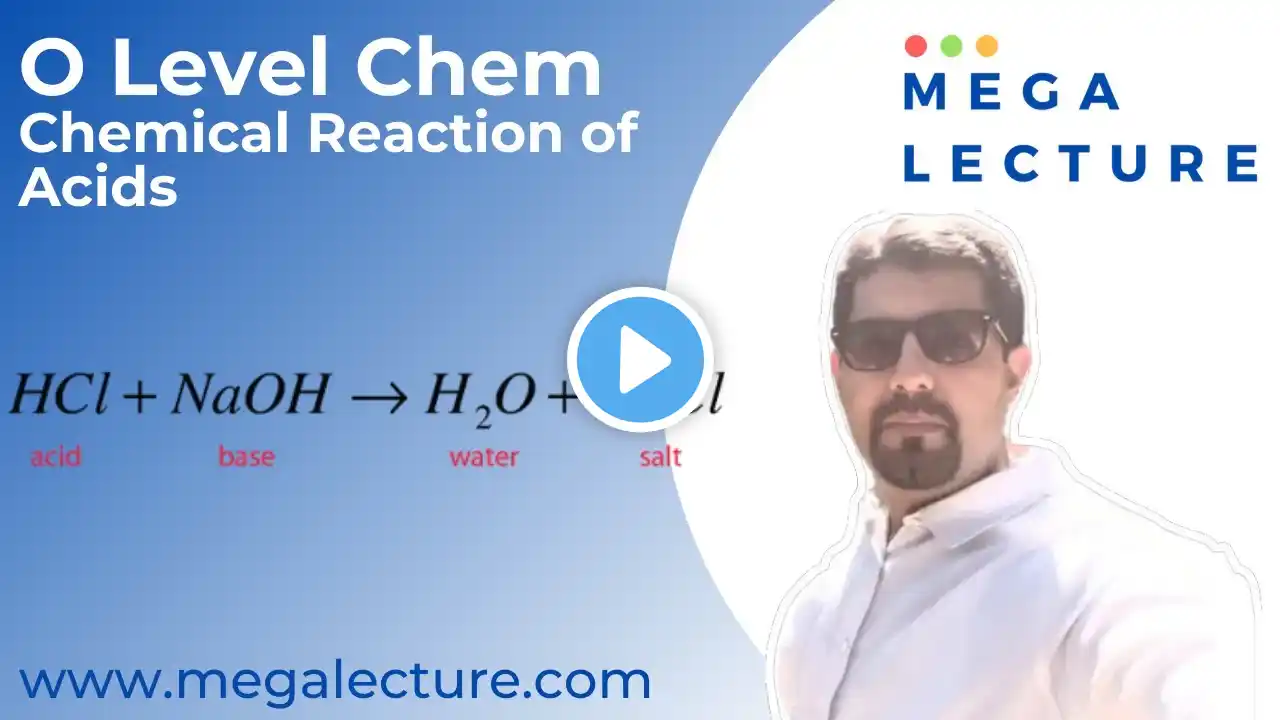
To join our Free Trial Lesson, Join our Whatsapp Community Now! https://chat.whatsapp.com/CWSRaMi4i3w... or msg at +92 323 509 4443 To purchases recorded courses with notes https://megalecture.com/courses/ Live Class - O'level/IGCSE/GCSE Chemistry - Metals Part 2 For Free Live Demo Classes, Register at https://megalecture.com/live-subject-... Timestamps: 1:16 - Displacement of metals 27:19 - Extraction of iron Iron is extracted from iron ore in a huge container called a blast furnace. In this lecture we discuss the reaction in detail. Iron ores such as haematite contain iron(III) oxide, Fe2O3. The oxygen must be removed from the iron(III) oxide in order to leave the iron behind. Reactions in which oxygen is removed are called reduction reactions. Carbon is more reactive than iron, so it can displace iron from iron(III) oxide. Here are the equations for the reaction: Iron(III) oxide + carbon → iron + carbon dioxide 2Fe2O3(s) + 3C(s) → 4Fe(l) + 3CO2(g) In this reaction, the iron(III) oxide is reduced to iron, and the carbon is oxidised to carbon dioxide. In the blast furnace, it is so hot that carbon monoxide can be used, in place of carbon, to reduce the iron(III) oxide: iron(III) oxide + carbon monoxide → iron + carbon dioxide Fe2O3(s) + 3CO(s) → 2Fe(l) + 3CO2(g) Removing impurities The calcium carbonate in the limestone thermally decomposes to form calcium oxide. calcium carbonate → calcium oxide + carbon dioxide CaCO3(s) → CaO(s) + CO2(g) The calcium oxide then reacts with silica (sand) impurities in the haematite, to produce slag - which is calcium silicate. calcium oxide + silica → calcium silicate CaO(s) + SiO2(s) → CaSiO3(l) This reaction is a neutralisation reaction. Calcium oxide is basic (as it is a metal oxide) and silica is acidic (as it is a non-metal oxide). For more Video Lectures for O Levels, A Levels, IB Diploma, AP Courses & Edexcel: https://www.megalecture.com / megalecture For Zoom/Whiteboard Subject Experts and Tutors and Free Online Trial Classes, Contact: [email protected]