Live Class -O-level, IGCSE, GCSE Chemistry - Ionic Bonding - Properties of Ionic Compounds
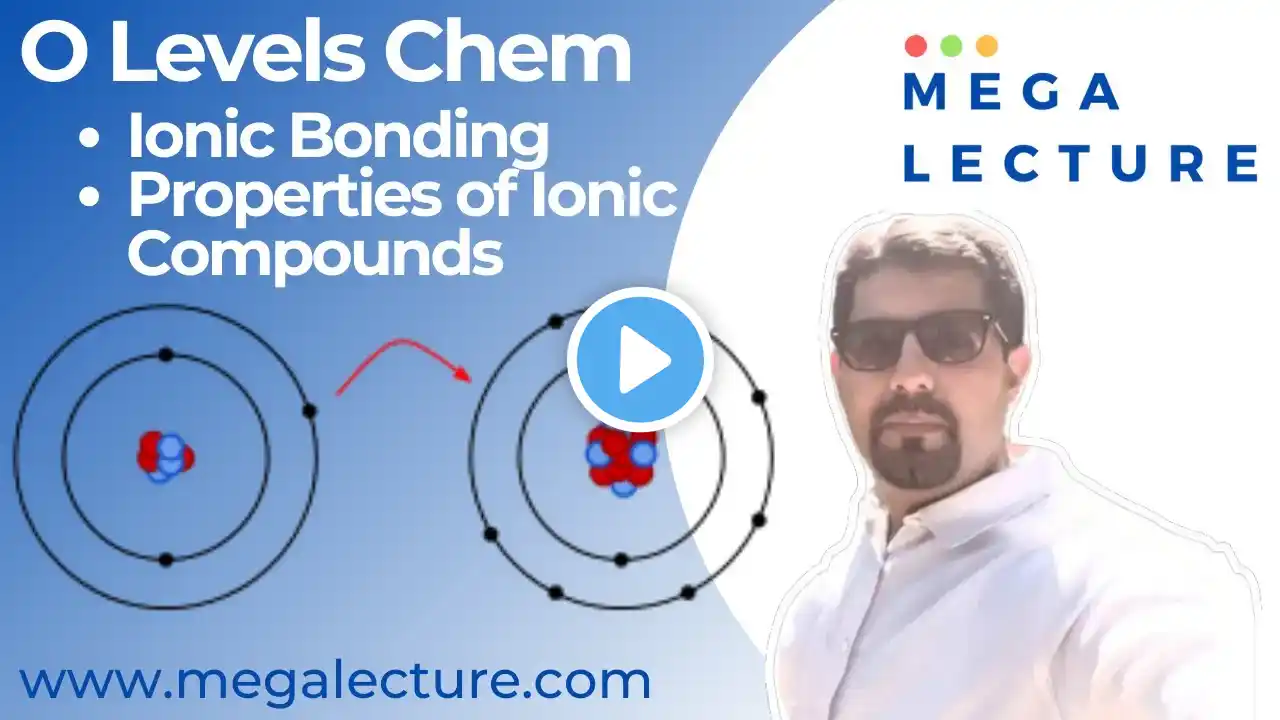
To join our Free Trial Lesson, Join our Whatsapp Community Now! https://chat.whatsapp.com/CWSRaMi4i3w... or msg at +92 323 509 4443 To purchases recorded courses with notes https://megalecture.com/courses/ Live Class -O-level, IGCSE, GCSE Chemistry - Ionic Bonding - Properties of Ionic Compounds For Free Live Demo Classes, Register at https://megalecture.com/live-subject-... In this lecture we will discuss ionic bonding which happens when electrons are lost and gained by two or more atoms. When atoms lose electrons they become positive ions. When atoms gain electrons they become negative ions. Ionic bonds are formed between metals and non - metals. Properties of ionic compounds: -They form giant ionic lattices. Conduct electricity when iin molten or aqueous state. Both these processes allow ions to move from place to place. Ionic compounds cannot conduct electricity in the solid state because their ions are held in fixed positions and cannot move. Since the electrostatic forces of attraction between oppositely charged ions are strong, their melting and boiling points are high. -They dissolve in water to some extent. For more Video Lectures for O Levels, A Levels, IB Diploma, AP Courses & Edexcel: https://www.megalecture.com / megalecture For Zoom/Whiteboard Subject Experts and Tutors and Free Online Trial Classes, Contact: [email protected]