Elevation in Boiling Point| Class 12 Chem.| Colligative Properties| #chemistry NEET| JEE| JEE Mains|
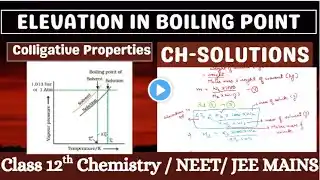
📘 Elevation in Boiling Point | Colligative Properties | Class 11 Chemistry In this video, we explain the concept of Elevation in Boiling Point, an important colligative property of solutions in physical chemistry. You will learn why the boiling point of a solvent increases when a non-volatile solute is added to it and how this phenomenon depends on the number of solute particles, not their nature. 🔍 Topics covered in this video: *Meaning of elevation in boiling point *Effect of non-volatile solute on vapour pressure *Relationship between boiling point and vapour pressure *Mathematical expression: ΔTb = Kb × m *Explanation of ebullioscopic constant (Kb) *Graphical representation 🎯 This video is useful for: Class 12 CBSE & State Board students NEET & JEE aspirants Chemistry beginners who want conceptual clarity 📌 Watch till the end to understand the concept clearly with simple explanations and examples. 👍 If you find this video helpful, don’t forget to Like, Share & Subscribe to the channel for more chemistry concepts explained in an easy way.


















