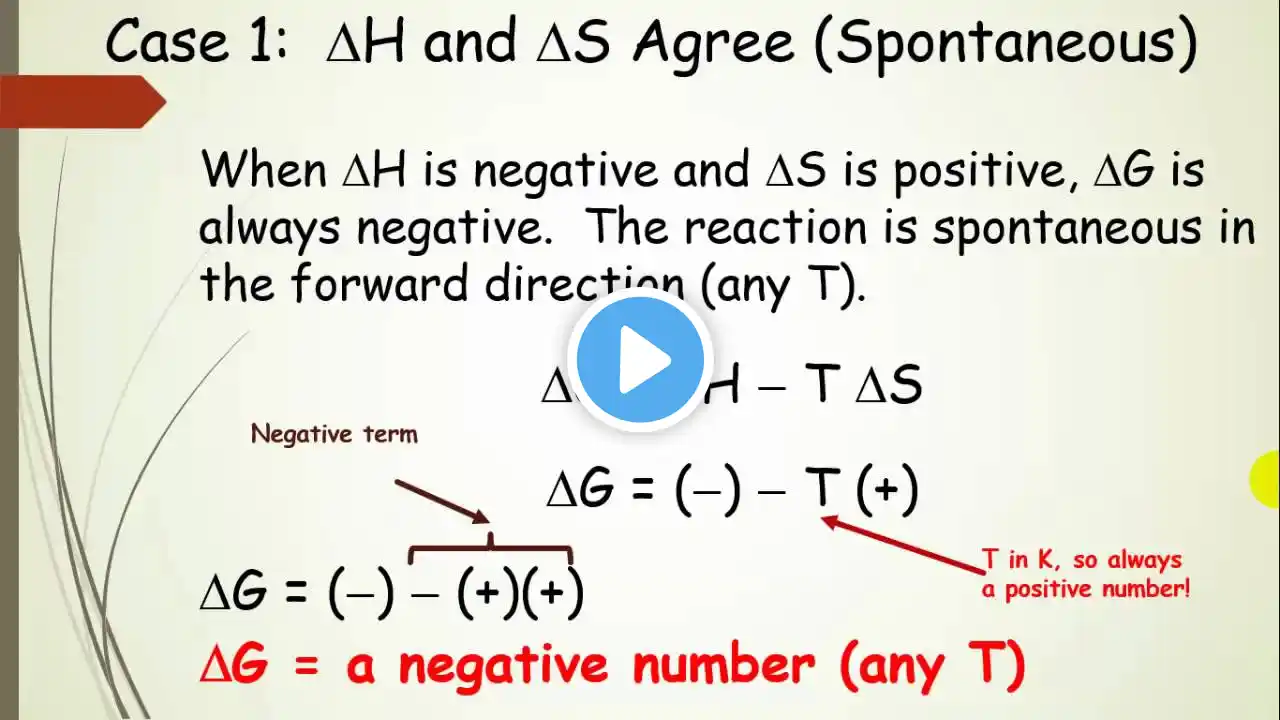
Predict if a Reaction is Spontaneous with Thermodynamic Signs
Given Enthalpy and Entropy signs and using Gibbs Free Energy Equation, predict if the reaction will be spontaneous/thermodynamically favorable (-G) or non-spontaneous/thermodynamically unfavorable (+G). Instagram: Lean.Think Website: LeanThink.org