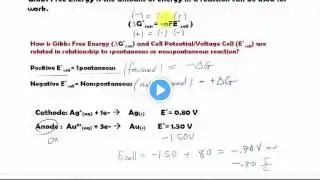
How to determine the sign of Gibbs Free Energy - Real Chemistry
In this video you will be introduced to spontaneity and learn how to use Gibbs free energy to determine if a chemical reaction is spontaneous, and at what temperatures. A negative Gibbs free energy indicates a spontaneous reaction, while a positive Gibbs free energy indicates a non-spontaneous reaction. Gibbs free energy depends on both the entropy and enthalpy change of a system as well as temperature.