How Does The Ideal Gas Constant (R) Relate To Other Gas Laws? - Chemistry For Everyone
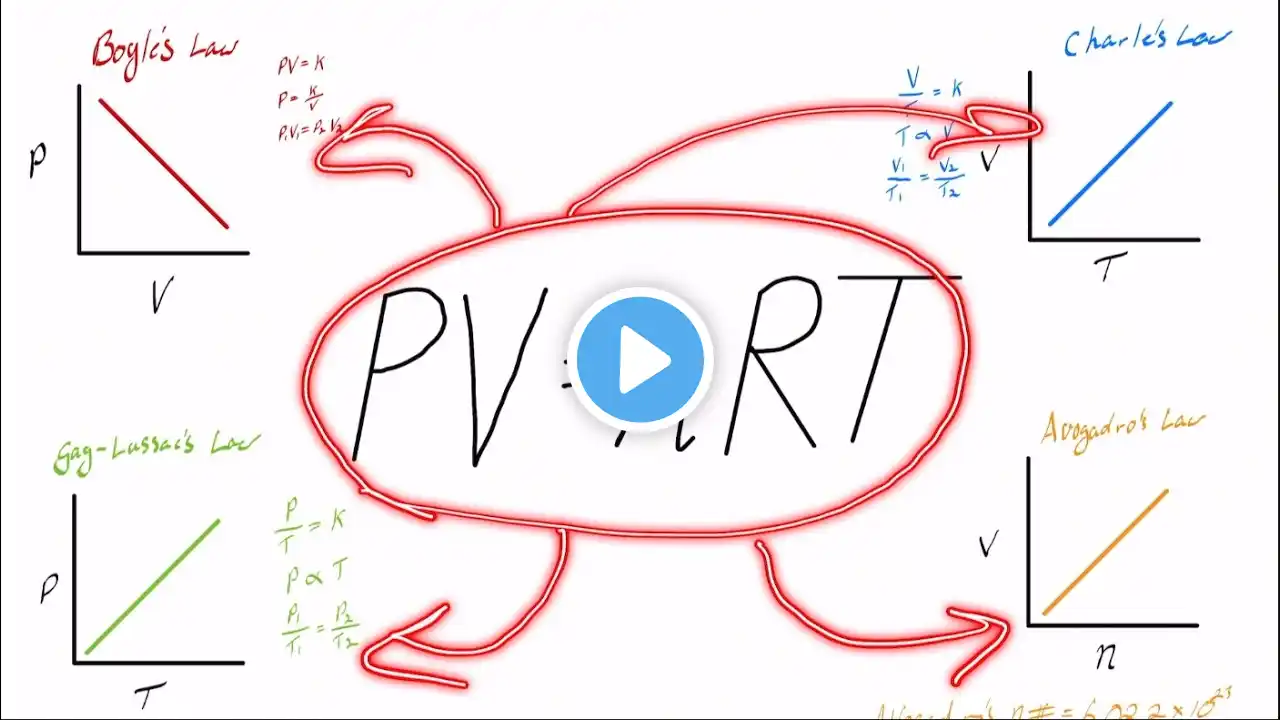
How Does The Ideal Gas Constant (R) Relate To Other Gas Laws? In this informative video, we’ll discuss the Ideal Gas Constant and its relationship with various gas laws. The Ideal Gas Constant, denoted as R, plays a significant role in the Ideal Gas Law, which is fundamental in understanding gas behavior. We will break down how R integrates concepts from Boyle’s Law, Charles’s Law, and Avogadro’s Law, showing how these principles interact in a gas system. This video will also highlight the universality of R, which has the same value for all ideal gases, making it applicable across a wide range of scenarios. Additionally, we will touch on how R connects to the Boltzmann Constant, bridging the gap between macroscopic and microscopic gas behavior. For those interested in practical applications, we’ll explain how to derive the specific gas constant from R for particular gases, which is essential in fields like engineering and atmospheric science. Lastly, we will address how real gases can behave differently under certain conditions and the corrections that can be applied while still using R as a baseline constant. Join us for this informative discussion, and subscribe to our channel for more engaging content on chemistry and gas behavior. ⬇️ Subscribe to our channel for more valuable insights. 🔗Subscribe: https://www.youtube.com/@Chemistry-Fo... #IdealGasLaw #GasLaws #Chemistry #Physics #BoylesLaw #CharlesLaw #AvogadrosLaw #GasBehavior #ChemistryEducation #Engineering #AtmosphericScience #BoltzmannConstant #GasConstant #RealGases #ScienceExplained About Us: Welcome to Chemistry For Everyone, your go-to destination for exploring the fascinating world of chemistry and materials science! Our channel is dedicated to making complex concepts accessible and enjoyable for everyone, from curious beginners to seasoned enthusiasts.