What Is Pressure (P) In The Ideal Gas Law? - Chemistry For Everyone
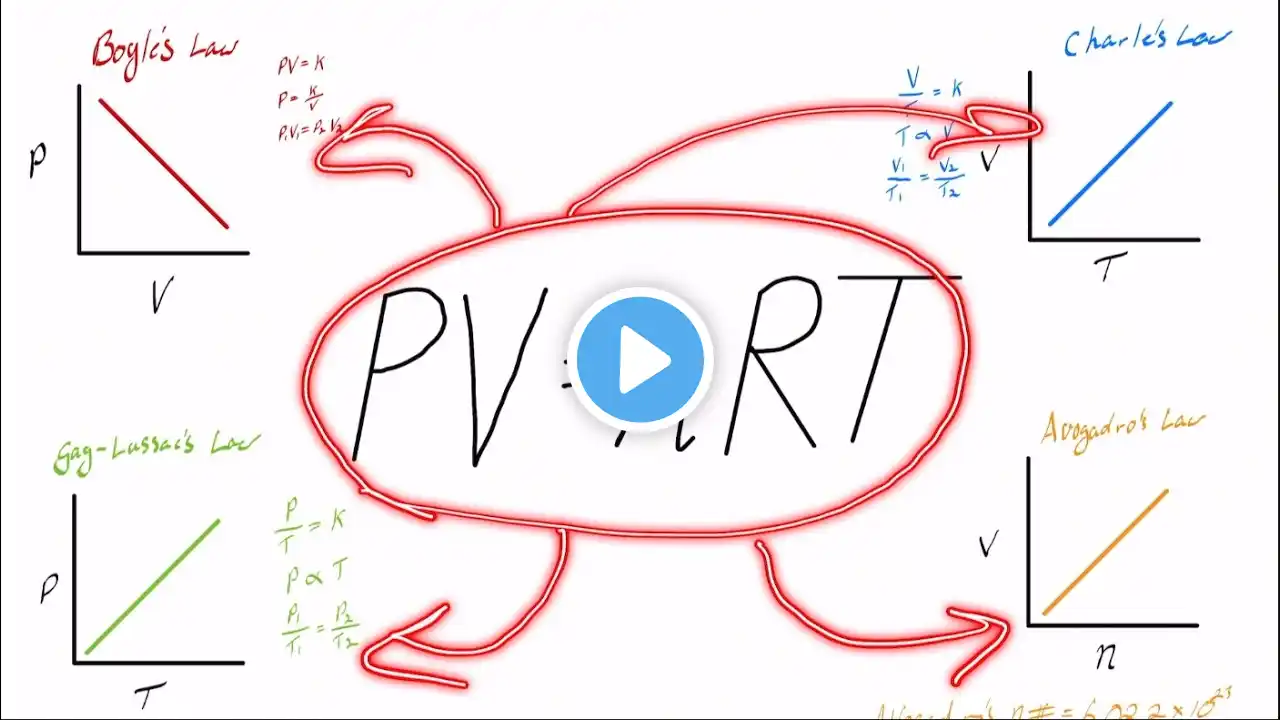
What Is Pressure (P) In The Ideal Gas Law? Have you ever wondered what causes gases to exert force on their containers and how this influences everyday situations? In this informative video, we'll explain everything you need to know about pressure in gases. We'll start by defining what pressure is and how it results from the constant movement and collisions of gas particles inside a container. You'll learn how the frequency and force of these collisions determine the pressure exerted on the container walls. We’ll also discuss how pressure relates to other properties of gases, such as volume, temperature, and the number of particles, as described by the ideal gas law. Whether you're inflating a balloon or working with industrial equipment, understanding how pressure behaves helps in predicting and controlling gas behavior. We'll explain how increasing temperature or adding more gas molecules impacts pressure, and why decreasing the volume of a container can lead to higher pressure. Additionally, we’ll review the units used to measure pressure, like atmospheres and kilopascals, and why these measurements are important for scientists and engineers. Knowing how pressure works is essential for a variety of applications, from everyday life to complex industrial processes. Join us for this clear and straightforward explanation, and subscribe to our channel for more helpful chemistry lessons. ⬇️ Subscribe to our channel for more valuable insights. 🔗Subscribe: https://www.youtube.com/@Chemistry-Fo... #GasPressure #IdealGasLaw #ChemistryBasics #PhysicsOfGases #PressureUnits #GasBehavior #ChemicalReactions #ScienceEducation #Physics #ChemistryHelp #GasLaws #PressureInGases #ScienceExplained #ChemistryForBeginners #EducationalVideo About Us: Welcome to Chemistry For Everyone, your go-to destination for exploring the fascinating world of chemistry and materials science! Our channel is dedicated to making complex concepts accessible and enjoyable for everyone, from curious beginners to seasoned enthusiasts.