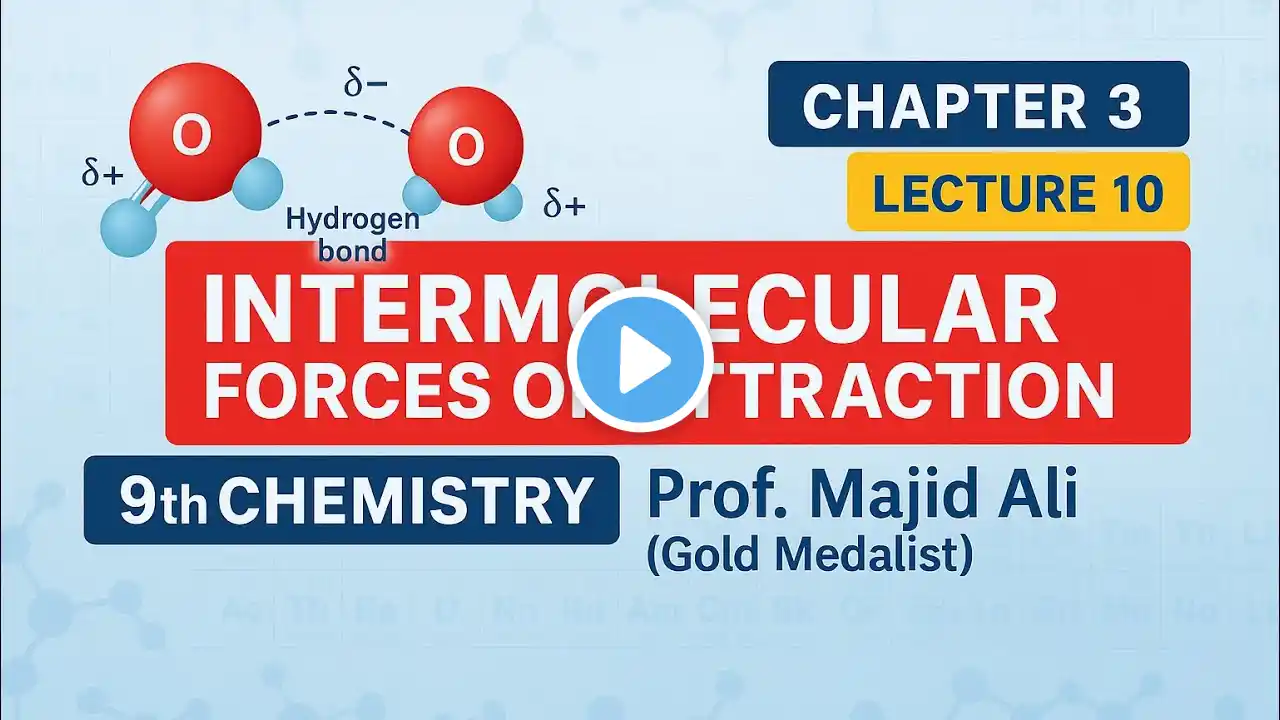
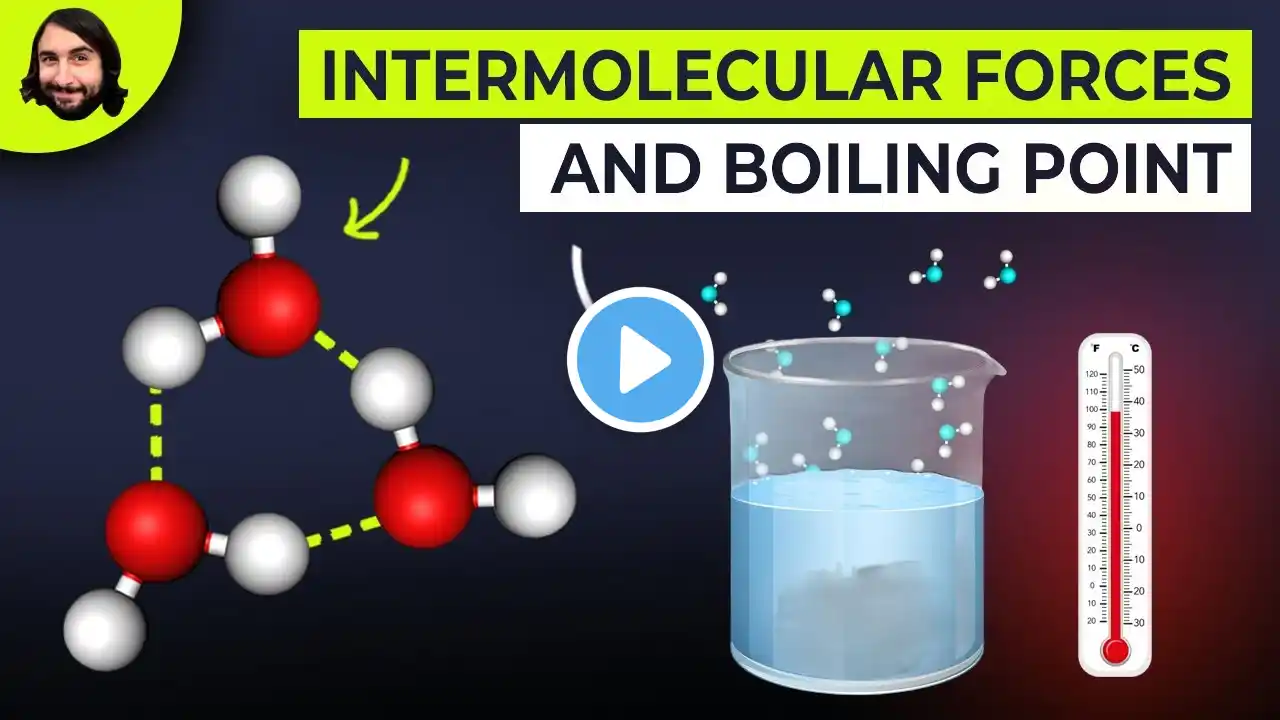
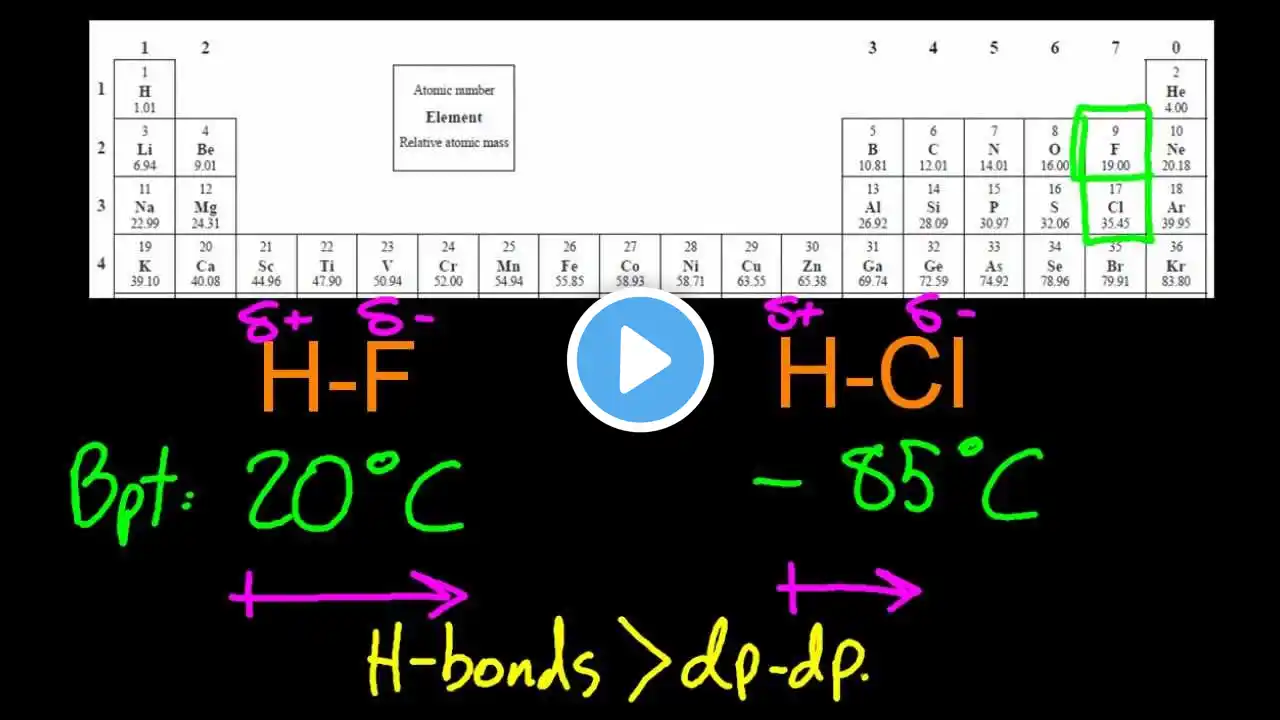
How do inter- and intramolecular forces affect boiling points?
Intermolecular Forces and Boiling Points 1. Molecules stickier need more heat 2. Hydrogen bonds raise boiling points 3. Dipoles increase melting and boiling 4. Ionic charges give very high points 5. Branching reduces surface contact Common Mistakes About Molecular Forces 1. Assuming size is the only factor 2. Forgetting branching affects surface area 3. Treating ionic and covalent same Visit https://www.gumballdegree.com to learn more. Want to learn more about this topic? Visit https://www.GumballDegree.com/how-do-... #intermolecularforces, #hydrogenbonding, #boilingpoints, #meltingpoints, #molecularpolarity, #branchingeffects, #ionicvs covalent, #dipoleinteractions, #physicalproperties, #chemistryconcepts