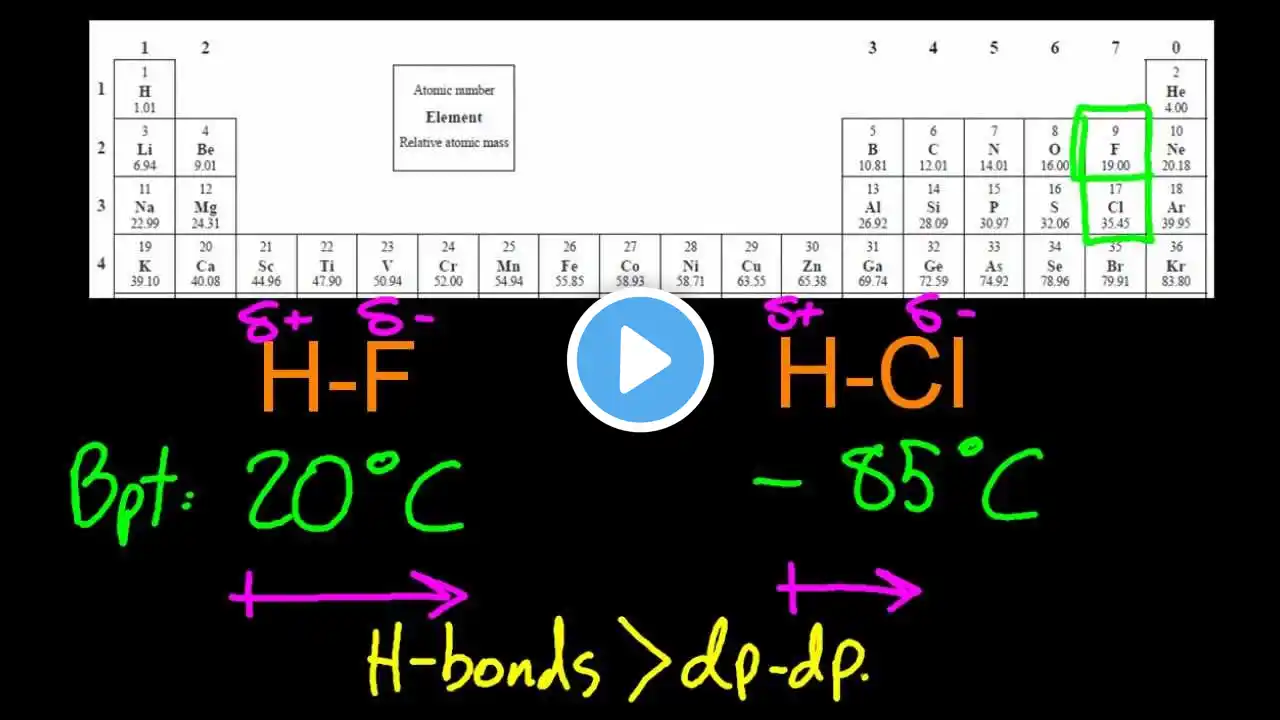
4.3.2 Describe & explain how intermolecular forces affect boiling points IB Chemistry SL
Intermolecular forces must be broken to boil a substance. Hydrogen bonds are the strongest intermolecular force, then dipole-dipole attractions and the weakest is Van Der Waals.