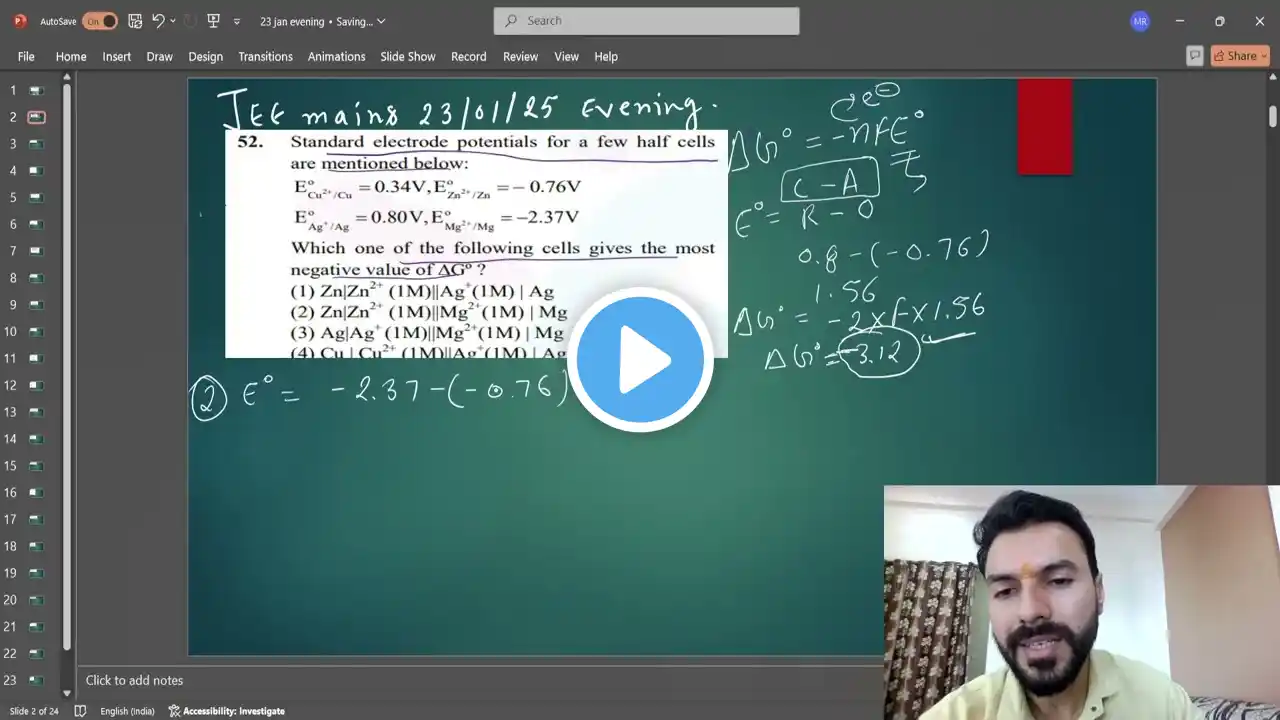
Standard electrode potentials for a few half cells are mentioned below:
Gibbs free energy change (ΔG°), standard cell potential (E°cell), and electrochemical cells are the key concepts for this question. The relationship between ΔG° and E°cell is given by the formula: ΔG° = -nFE°cell, where n is the number of moles of electrons transferred, F is the Faraday constant, and E°cell is the standard cell potential. To obtain the most negative ΔG°, the E°cell should be the most positive. E°cell is calculated by subtracting the standard reduction potential of the anode (oxidation half-cell) from the cathode (reduction half-cell), i.e., E°cell = E°cathode - E°anode. A higher value of E°cell indicates a more spontaneous cell reaction and thus a more negative value of ΔG°. To find the right cell, determine which combination of anode and cathode gives the maximum positive E°cell. In this problem, each cell notation follows the standard format: Anode | Anode solution || Cathode solution | Cathode. The metal on the left undergoes oxidation, and the one on the right undergoes reduction. By calculating E°cell for each cell using the given potentials, we can find which cell has the highest E°cell and therefore the most negative ΔG°. This method is essential in predicting cell spontaneity and designing batteries or galvanic cells. Question Statement: Standard electrode potentials for a few half cells are mentioned below: E°(Cu^2+ / Cu) = +0.34 V E°(Zn^2+ / Zn) = -0.76 V E°(Ag^+ / Ag) = +0.80 V E°(Mg^2+ / Mg) = -2.37 V Which one of the following cells gives the most negative value of ΔG°? (1) Zn | Zn^2+ (1M) || Ag^+ (1M) | Ag (2) Zn | Zn^2+ (1M) || Mg^2+ (1M) | Mg (3) Ag | Ag^+ (1M) || Mg^2+ (1M) | Mg (4) Cu | Cu^2+ (1M) || Ag^+ (1M) | Ag