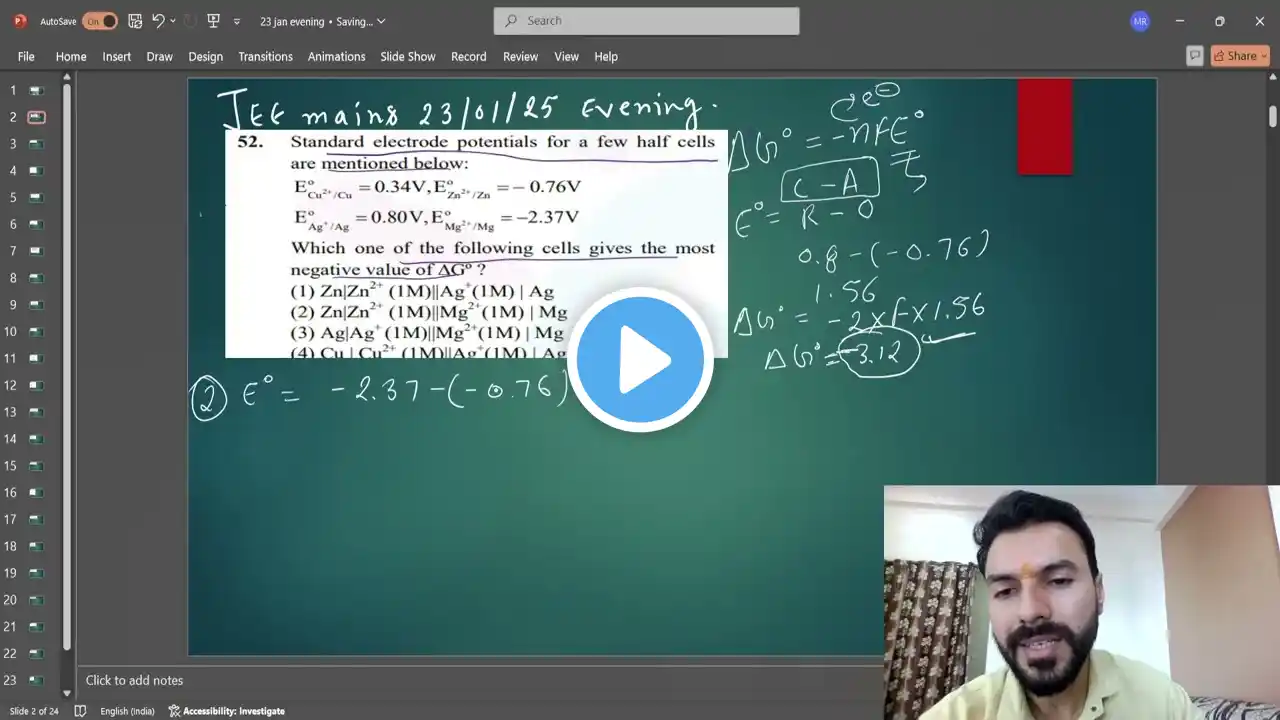
JEE mains 2025 PYQ Standard electrode potentials for a few half cells are mentioned below: E0 Cu2+ /
Standard electrode potentials for a few half cells are mentioned below: E0 Cu2+ /Cu+ = 0.34V Zn2+/Zn E0 = -0.76V EoAg+ /Ag= 0.80V, E Mg2+ /Mg E0 =2.37VWhich one of the following cells gives the most negative value of ∆Gº ? (1) Zn|Zn2+ (1M)||Ag+(1M) | Ag(2) Zn|Zn2+ (1M)||Mg2+(1M) | Mg(3) Ag|Ag+ (1M)||Mg2+(1M) | Mg(4) Cu | Cu2+ (1M)||Ag+(1M) | Ag Jee mains 23 january 2025 Evening shift question paper solution #education #jeeproblems #jeemain2025 #jeebatch #chemicalformula