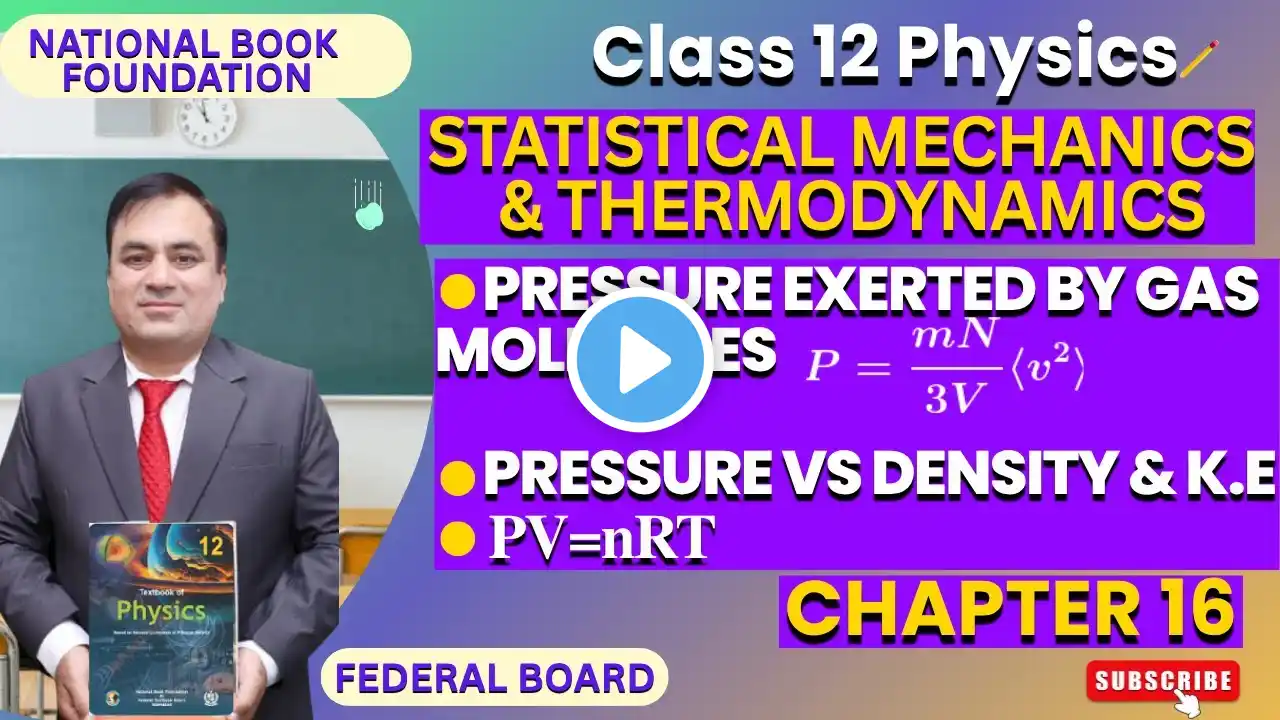
Ideal Gas Law Explained | PV = nRT | Chemistry & Physics Tutorial
In this video, we break down the Ideal Gas Law (PV = nRT) step by step in a clear and simple way. You’ll learn what each variable means, how pressure, volume, temperature, and number of moles are related, and how to apply the formula to common chemistry and physics exam questions. This lesson is perfect for high school chemistry and physics students, especially those preparing for tests, quizzes, and final exams. 📘 Topics covered: What the Ideal Gas Law is Meaning of P, V, n, R, and T When to use PV = nRT Units and common mistakes Example problems explained clearly 🎓 Need extra help? Get private one-on-one tutoring in math, physics, and chemistry: 👉 https://www.theprivatetutor.ca #chemistry #ibchemistry #pv=nrt #ocdsb #wqsb #idealgaslaw #gaslaws #grade11chemistry