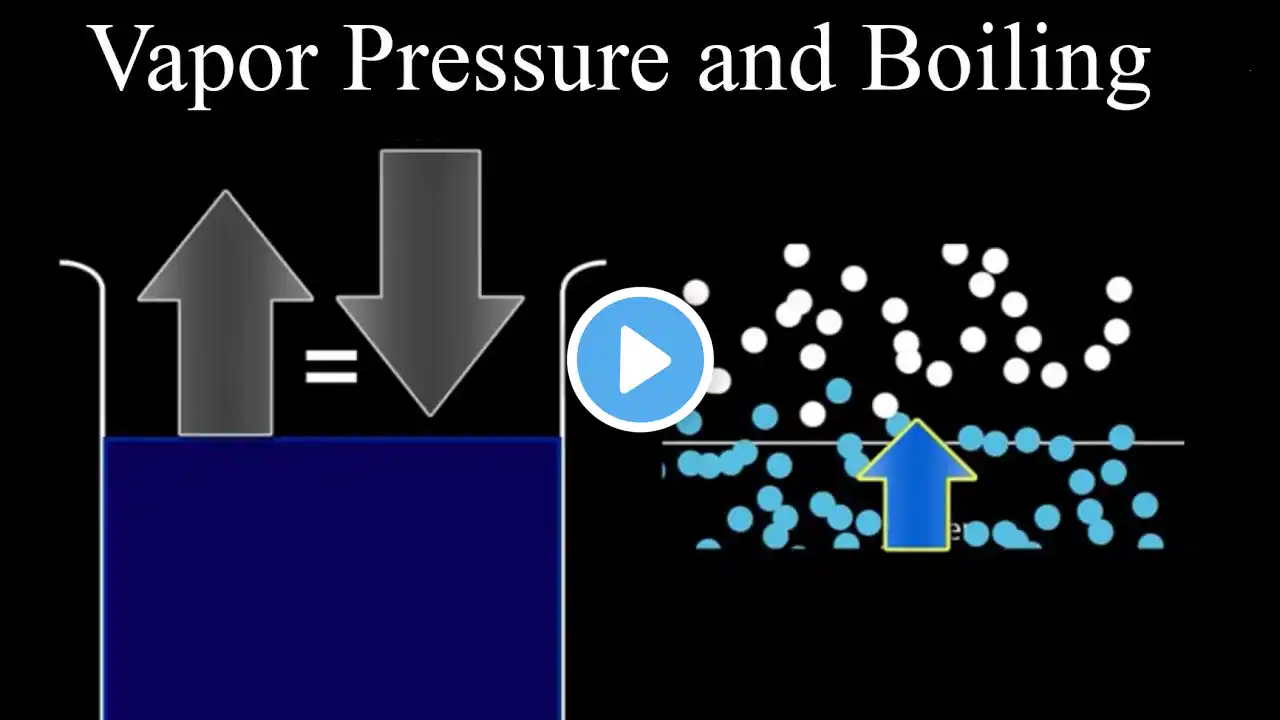
Why does water boil at high altitudes at lower temperatures?
Everyday experience shows that water begins to boil at about 100 °C (212 °F) under normal conditions. However, on Mount Everest, at an altitude of about 8850 meters, the water boils at a temperature of around 71 °C (160 °F). Thus, in this video we deal with the question why water boils at high altitudes already at lower temperatures. 00:00 Why does water boil at high altitudes at lower temperatures? 01:07 Explanation with the particle model 02:58 Pressure cooker 04:30 Experiment