Ideal Gas Equation & Dalton’s Law Explained | pV = nRT Made Super Easy! | Class 11 Chemistry
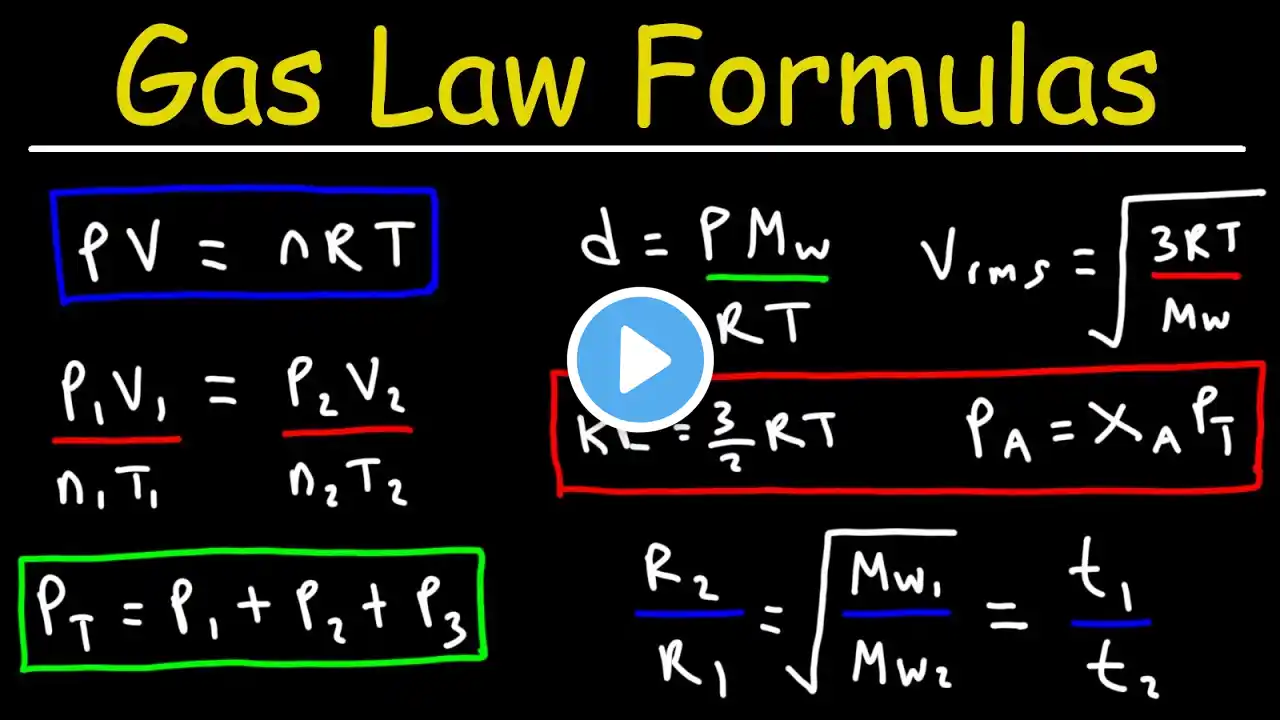
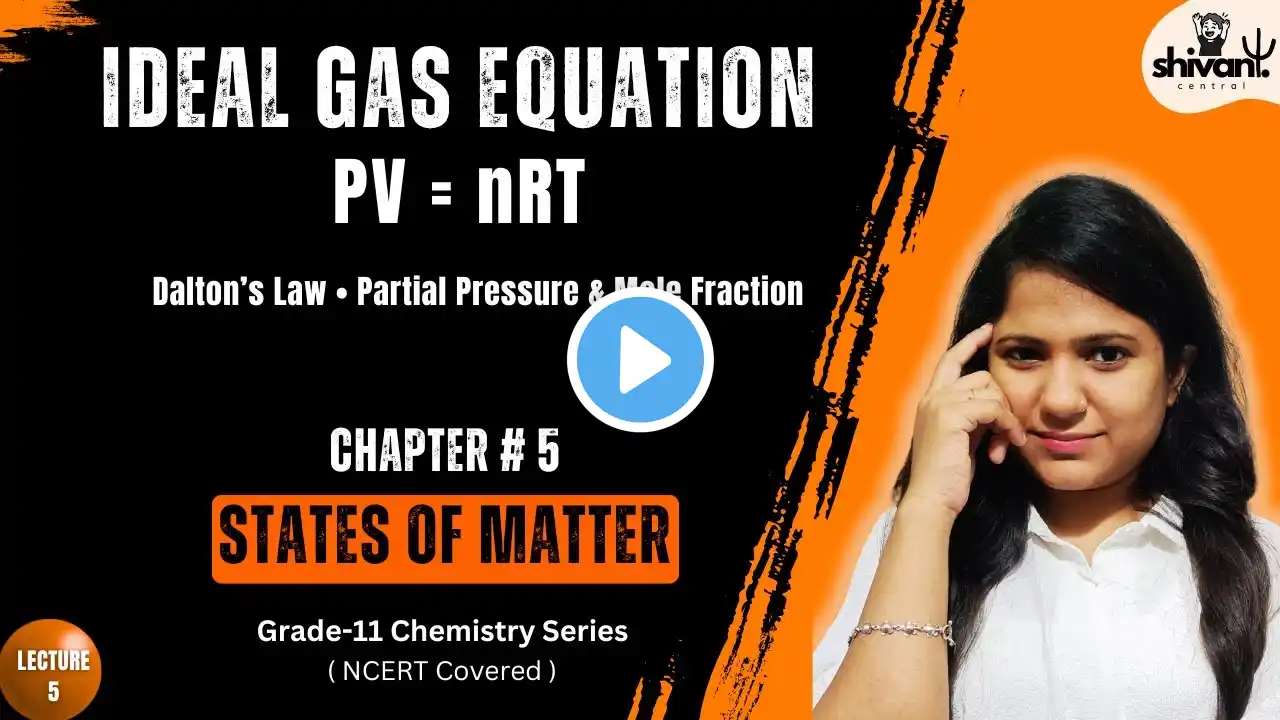
In this educational video for Class 11 Chemistry, we explore the fundamentals and applications of the Ideal Gas Equation (PV = nRT). We also explore Dalton's Law of Partial Pressures and how mole fraction and partial pressure help analyse mixtures of gases. This lesson clears the path to solving problems in gaseous state chemistry and lays the foundation for thermodynamics and real-gas behaviour. Topics Covered Derivation & explanation of the Ideal Gas Equation (PV = nRT) Applications of the Ideal Gas Equation in calculating pressure, volume, number of moles and temperature changes Dalton’s Law of Partial Pressures: definition and formula The concept of mole fraction and how it relates to partial pressures Worked examples applying the Ideal Gas Equation and Dalton’s Law to gas mixtures Key assumptions, limitations and real‐gas deviations Timestamps 00:00 – Intro 00:27 – Ideal Gas Equation 05:18 – Applications of the Ideal Gas Equation 06:18 - Limitations 06:55 – Dalton’s Law of Partial Pressures & Mole Fraction 10:41 - Applications 12:37 – Example problem 15:08 – Summary and Takeaway What You Will Learn How to apply the Ideal Gas Equation to real problems involving gases The relationship between mole fraction and partial pressure in gas mixtures How Dalton’s Law helps resolve pressures in multi‐component gas systems Why gases deviate from ideal behaviour under certain conditions Practical insights for CBSE, ISC, JEE and NEET exams Who Should Watch Class 11 Chemistry students (CBSE, ISC and State Boards) Aspirants preparing for JEE / NEET needing conceptual clarity in gas laws Anyone looking to strengthen their understanding of gaseous state chemistry and problem solving #IdealGasEquation #PVnRT #DaltonsLaw #PartialPressure #MoleFraction #GasLaws #Class11Chemistry #ChemistryTutorial #GaseousState #ChemistryMadeEasy #JEEChemistry #NEETChemistry …………………………………………………. 💡 Smart Tip to Watch This Video: For the best learning outcome – Step 1: Watch at normal speed to fully grasp the concept with examples. Step 2: Re-watch at 1.5x–2x speed. This acts like active revision, helping your brain strengthen memory recall and retain formulas & definitions more effectively. We believe education should be accessible, simple, and inspiring for every learner. Our goal is to help you build strong concepts, develop curiosity, and grow with confidence in your studies and beyond. 📌 For more learning updates and resources, connect with us on: Instagram: / shivanicentral Facebook: https://www.facebook.com/profile.php?... …………………………………………………. Disclaimer: This video is created solely for educational and informational purposes. We do not intend to infringe upon any copyright or intellectual property rights. If you believe that any copyrighted material has been used inappropriately, please contact us at [email protected]. We will review the matter promptly and take appropriate action