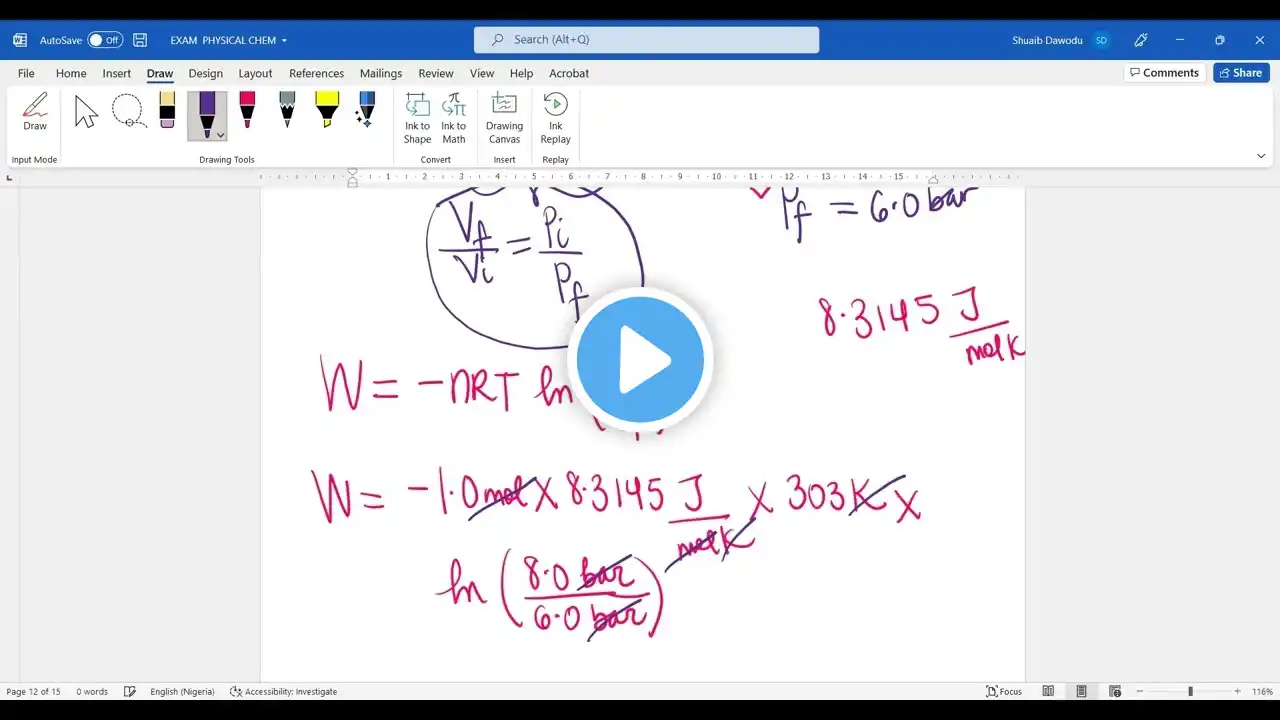
Student Exam Question: WORKDONE in an Isothermal Reversible Expansion
One mole of an ideal gas is contained in a cylinder-piston at 8.0bar and 303K. An isothermal and reversible expansion decreases the pressure of the gas to 6.0bar. How much work is done by the gas during the expansion?