How to solve examples on entropy of a thermodynamic system - SPPU paper solutions
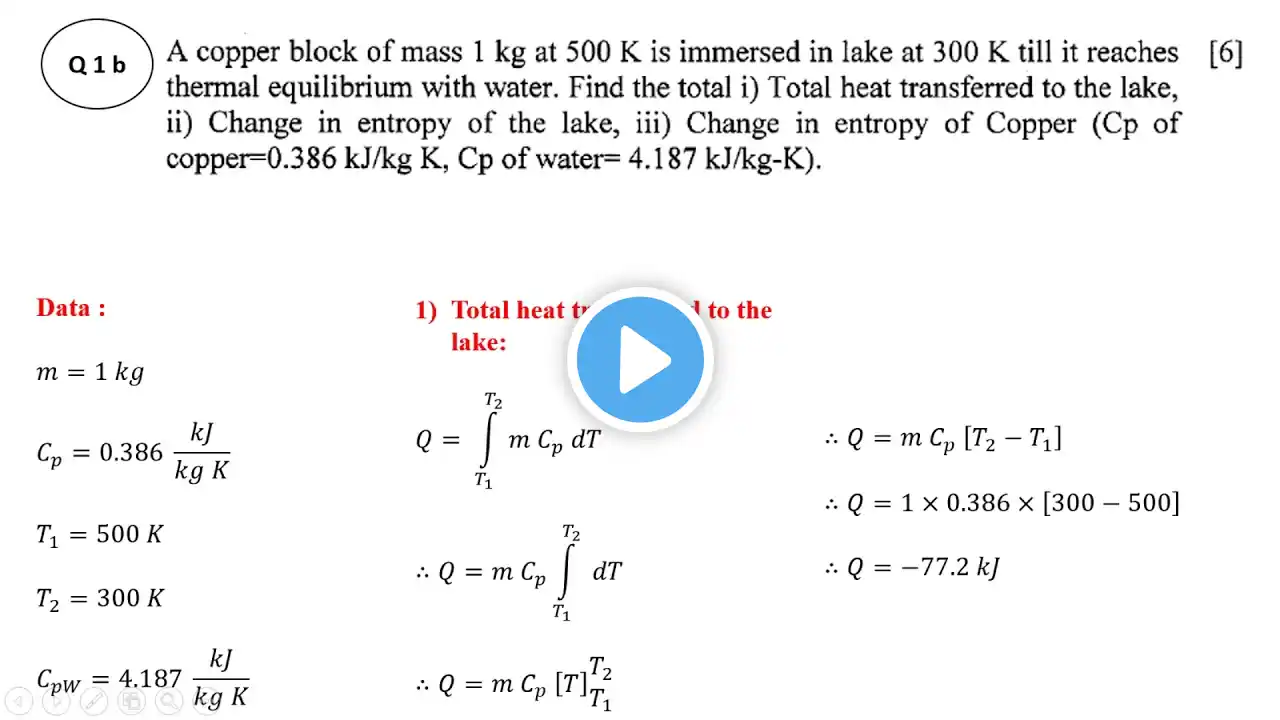
This video explains how to solve examples on entropy of a thermodynamic system. This example is taken from MAY 2018 question paper of Thermodynamics of Savitribai Phule Pune University (S.P.P.U.) The problem statement is as follows: A copper block of mass 1 kg at 500K is immersed in a lake of 300 K till it reaches thermal equilibrium with water. Find the : 1) Total heat transferred to the lake 2) Change in entropy of the lake 3) Change in entropy of copper (Cp of copper = 0.386 kJ/kgK, Cp of water = 4.187 kJ/kgK). Useful playlists: Cam profile - https://bit.ly/3vjpY7a Mechanics - https://bit.ly/3PClXmz Beam reaction & Graphic statics - https://bit.ly/3veMwWA SFD and BMD- Simply supported beam and cantilever beam - https://bit.ly/3PDzA4V SOM & Structural analysis videos - https://bit.ly/3OEqX8S Heat transfer - https://bit.ly/3PzxiUe Thermodynamics - https://bit.ly/3J5ZvQd Velocity & Acceleration diagram - https://bit.ly/3vj8zf0 Hydraulic circuit & pneumatic circuit working - https://bit.ly/3zAx1e4 Fluid mechanics - https://bit.ly/3ODuggg Dynamics of machinery playlist : https://bit.ly/3zyRakK SPPU Paper solutions -https://bit.ly/3S7dmcU Turbomachinery - https://bit.ly/3cyBftE I.C. Engine numericals -https://bit.ly/3veJf9K Machine Design - https://bit.ly/3zBjZgx Thermodynamics Excel Calculators - https://bit.ly/3cJBYbM Synthesis of mechanisms - https://bit.ly/3PFSCaD Finite Element Analysis (F.E.A.) - https://bit.ly/3PYtCex Mathematics - https://bit.ly/3JaAnHX Click the PayPal Donate Link below to support my YouTube Channel : https://paypal.me/RajanGosavi


















