Chemistry Class 12 Sem 4 Unit-4 Class 04 : Valence Bond Theory (VBT)
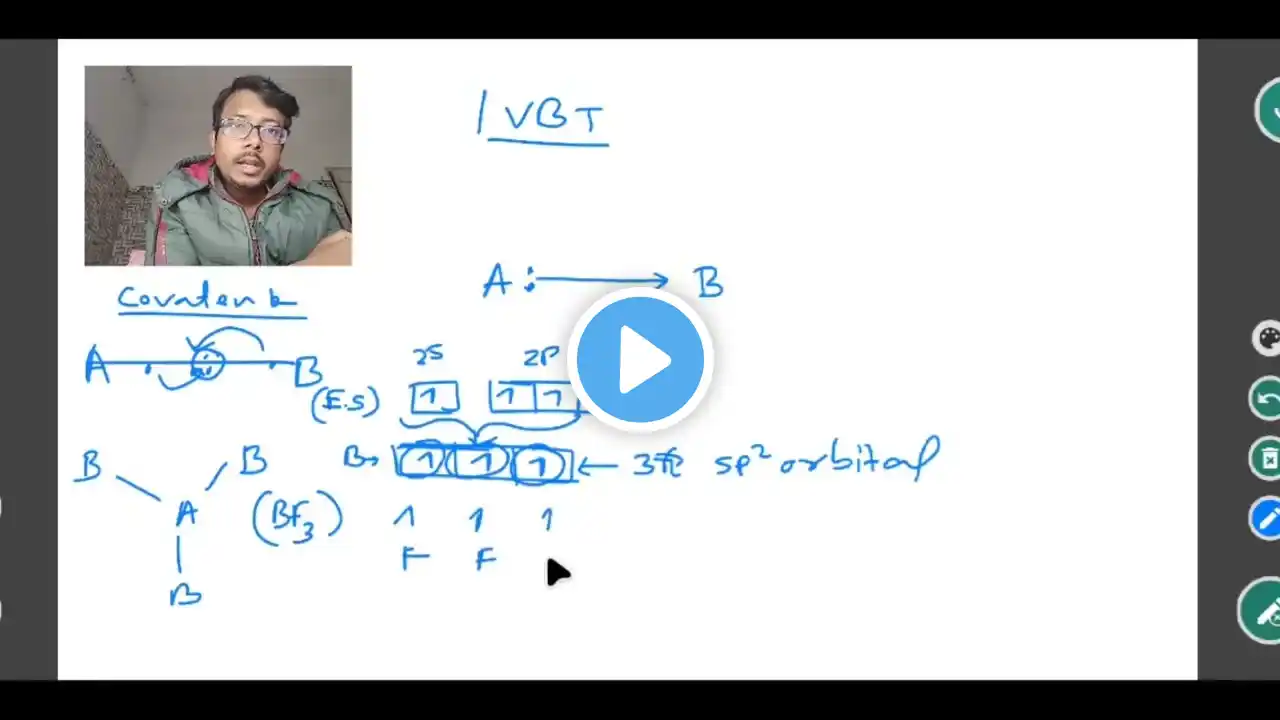
In this lecture, we dive deep into Valence Bond Theory (VBT), a crucial topic in Chapter 4: Coordination Compounds for Class 12 Chemistry. Understanding VBT is essential for predicting the geometry, hybridization, and magnetic properties of complex compounds. Whether you are preparing for Board Exams, JEE, or NEET, this session will simplify the most complex concepts of metal-ligand bonding. What You Will Learn: The Postulates of VBT: How metal ions provide vacant orbitals for ligand bonding. Hybridization States: Understanding sp^3, dsp^2, sp^3d^2, and d^2sp^3. Geometry Prediction: How to determine if a complex is Tetrahedral, Square Planar, or Octahedral. Inner vs. Outer Orbital Complexes: Why some complexes are "High Spin" and others are "Low Spin." Magnetic Behavior: How to calculate the spin-only magnetic moment (\mu) using the formula \mu = \sqrt{n(n + 2)}. Key Examples Covered: [Co(NH_3)_6]^{3+} (Diamagnetic, Inner Orbital) [CoF_6]^{3-} (Paramagnetic, Outer Orbital) [NiCl_4]^{2-} vs [Ni(CN)_4]^{2-} (Geometry differences) 📌 Practice Worksheet: [Link to Questions] Don't forget to: ✅ Subscribe for more Chemistry tutorials. ✅ Like the video if you found it helpful. ✅ Comment your doubts below—I'll be answering them! #Class12Chemistry #VBT #CoordinationCompounds #ChemistryLecture #JEEChemistry #NEETPreparation #ValenceBondTheory











