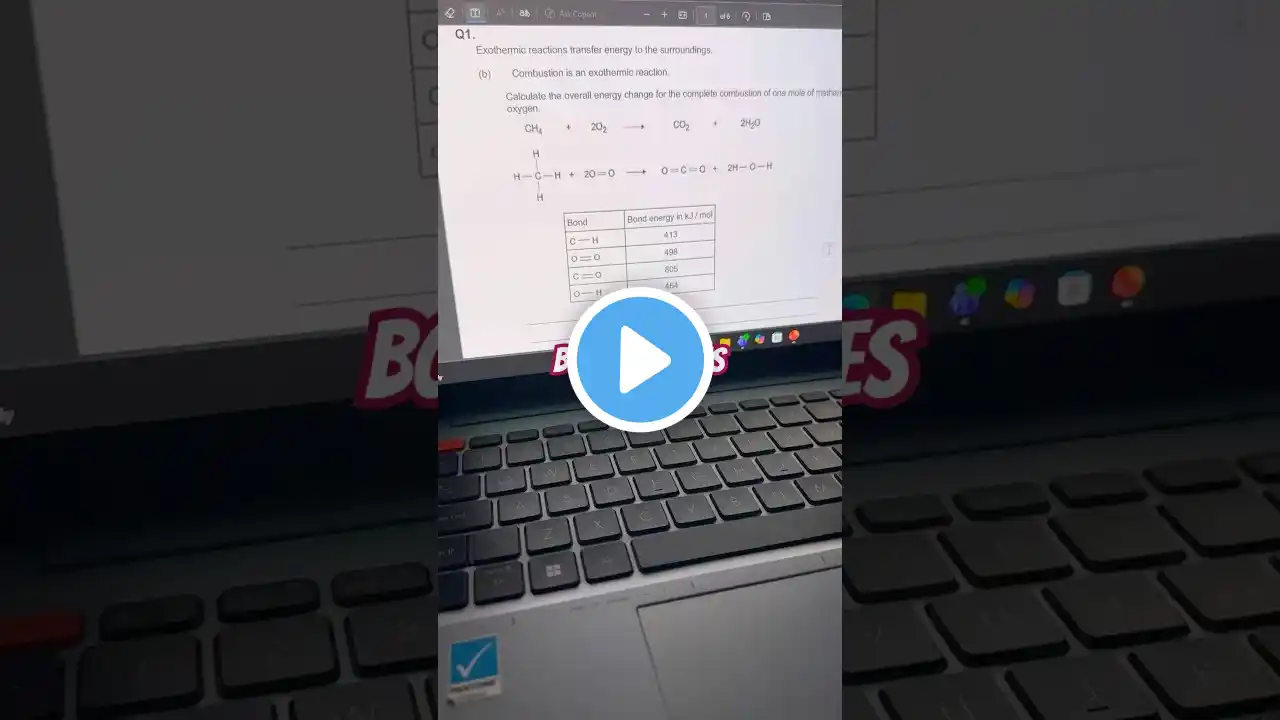
Bond Energy Calculations #chemistrypage #gcsechemistry #chemistry #gcse #gcsescience
👉 Energy in – Energy out = ΔH (Or: Bonds broken – Bonds formed) Here’s the quick method your examiner wants you to know: 1️⃣ Write the balanced equation 2️⃣ List the bonds broken (always + energy) 3️⃣ List the bonds formed (always − energy) 4️⃣ Add them up → final ΔH 5️⃣ State whether it’s endothermic or exothermic 💡 TOP TIP: If ΔH is negative, the reaction is exothermic. If ΔH is positive, it’s endothermic. Save this for your revision — and tag a friend who keeps mixing these up! 🤝📚 #GCSEChemistry #BondEnergies #GCSEScience #MissMalikScienceTutor #chemistryrevision