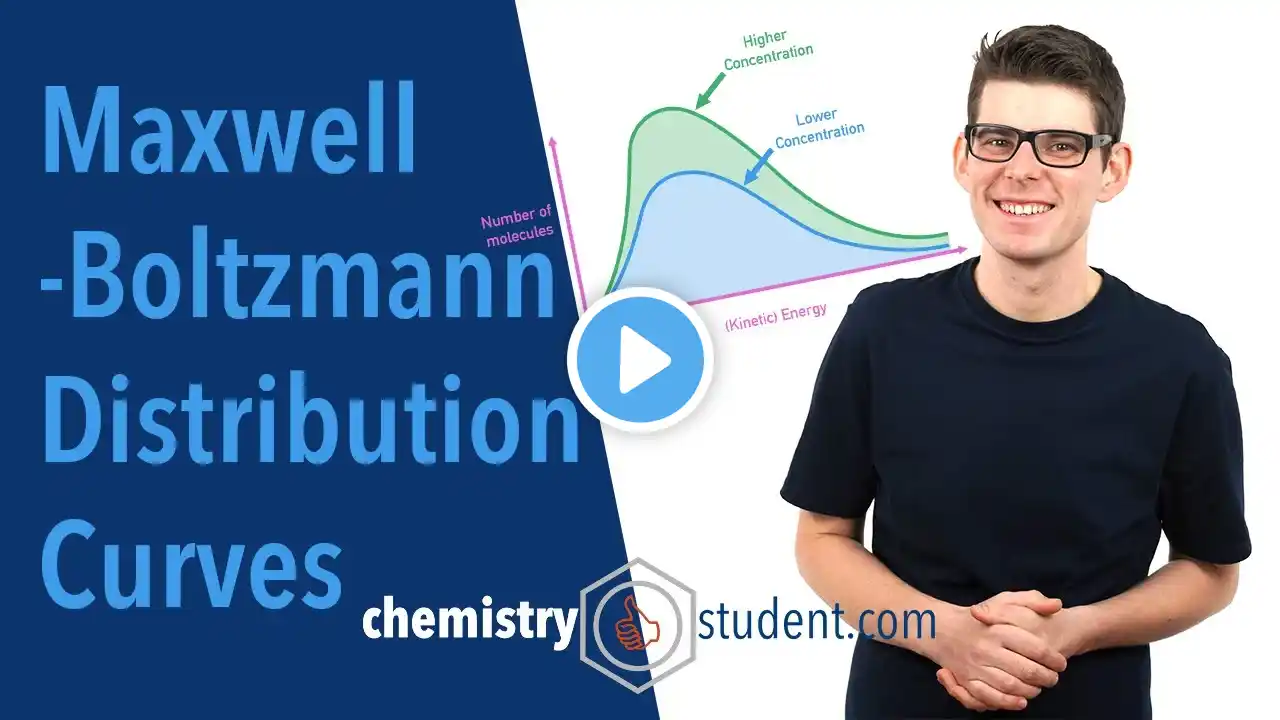
Maxwell-Boltzmann Distribution Curves (A-Level IB Chemistry)
Outlining Maxwell Boltzmann distribution curves and showing how they can be used to explain rates of reaction. Effect of temperature change, concentration change and the use of a catalyst are shown in terms of average energies of reacting particles. Recap: 00:36 Energy Distribution: 01:59 Maxwell-Boltzmann Distribution Curves: 03:21 Effect of Temperature Change: 07:15 Effect of Concentration Change: 08:37 Effect of a Catalyst: 10:00 Summary: 10:49 Pages on chemistrystudent.com: https://www.chemistrystudent.com/bolt... https://www.chemistrystudent.com/coll... Relevant Videos: Collision Theory and Activation Energy • Collision Theory and Activation Energy (A-... Thank you for watching - if you found the video useful, please like and subscribe!


















