How Do You Calculate Gibbs Free Energy Under Non-standard Conditions? - Thermodynamics For Everyone
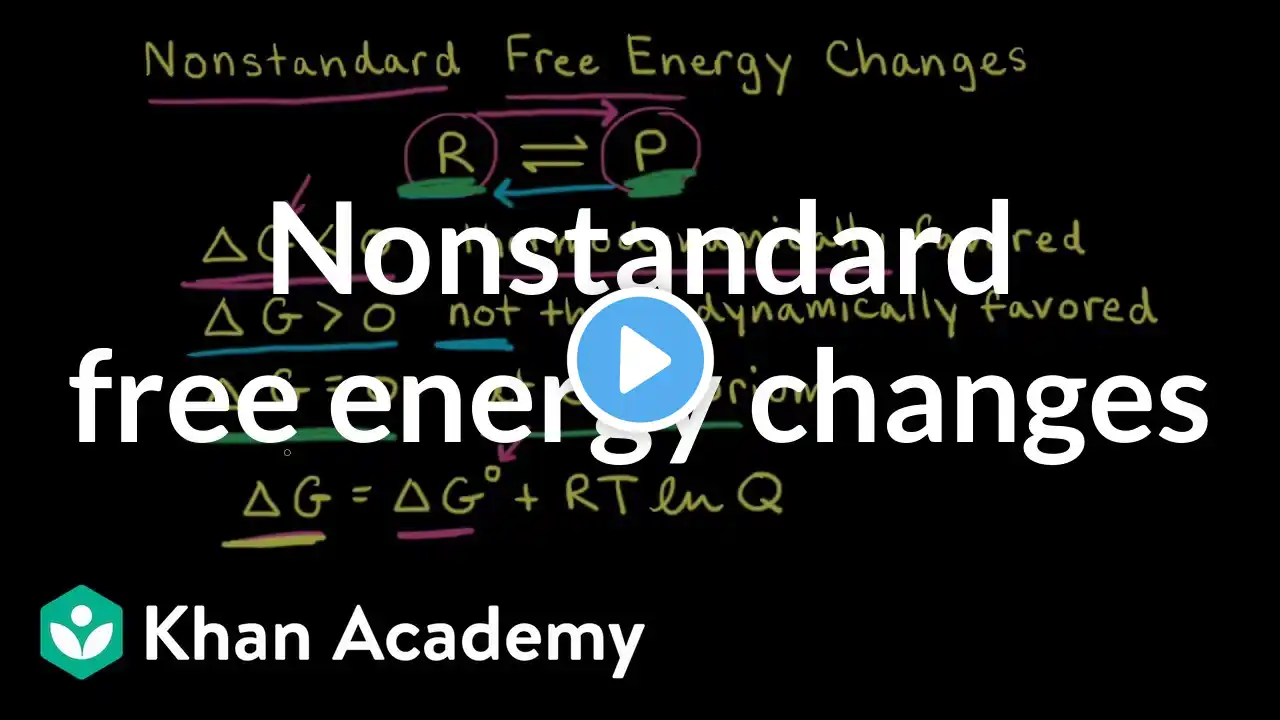
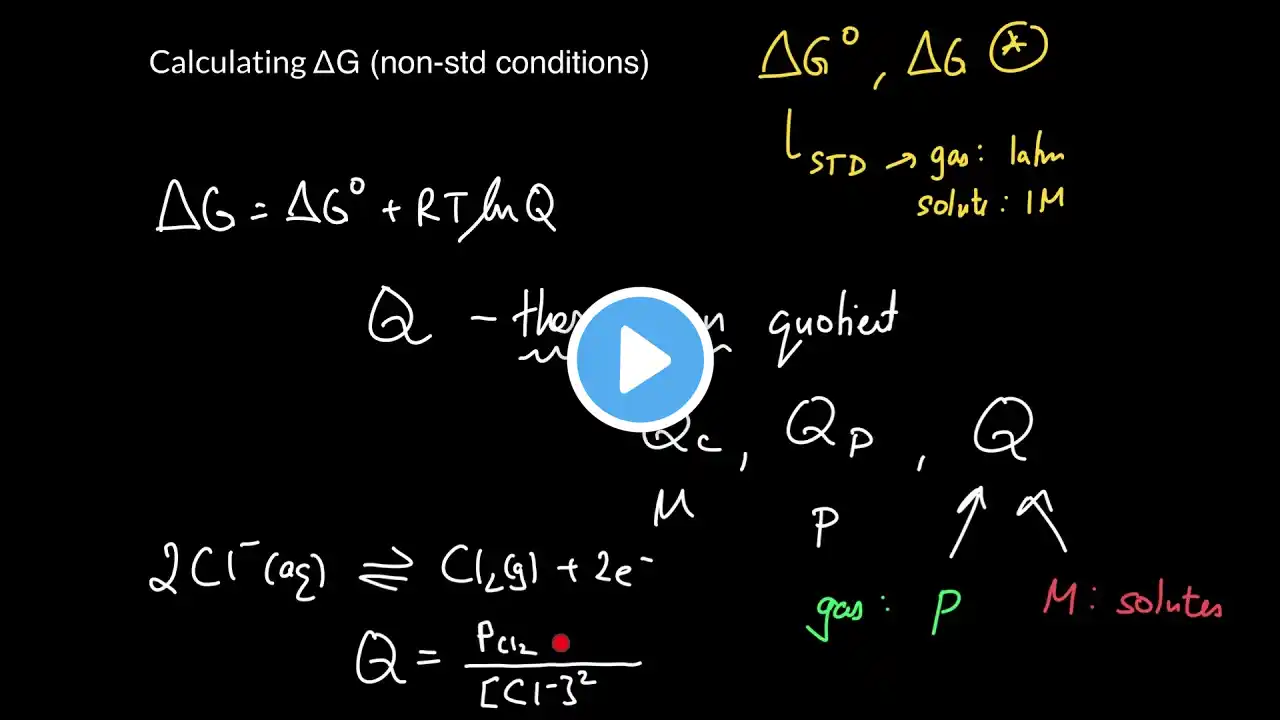
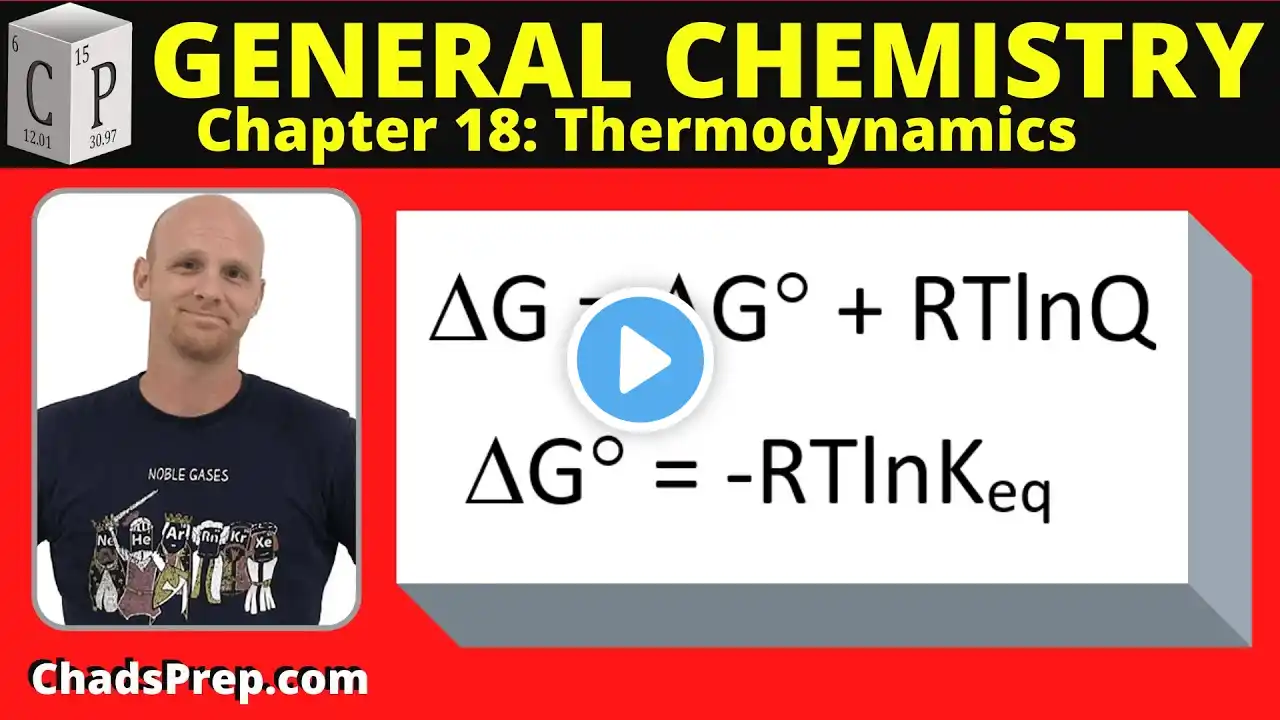
How Do You Calculate Gibbs Free Energy Under Non-standard Conditions? Have you ever wondered how scientists determine whether a chemical reaction will happen spontaneously? In this video, we’ll explain the process of calculating Gibbs Free Energy under real-world conditions. We’ll start by discussing what Gibbs Free Energy is and why it’s important for predicting reaction behavior. You’ll learn how to find the standard Gibbs Free Energy change, which serves as a baseline for your calculations. We’ll then introduce the reaction quotient, Q, which reflects the current state of the system based on actual concentrations or pressures. Next, we’ll walk through the formula that adjusts the standard Gibbs Free Energy to match real conditions, highlighting how temperature and the reaction quotient influence the outcome. We’ll explain what it means when ΔG is negative, positive, or zero, and how this relates to spontaneity and equilibrium. Additionally, we’ll connect these ideas to practical applications in biological systems and industrial processes, showing how changing conditions can affect reaction direction. Understanding this concept helps you predict whether a reaction needs extra energy to proceed or if it will happen naturally. Whether you’re a student, scientist, or curious learner, mastering this calculation is key to grasping thermodynamics beyond ideal scenarios. Join us to learn how to apply these principles effectively. ⬇️ Subscribe to our channel for more valuable insights. 🔗Subscribe: https://www.youtube.com/@Thermodynami... #Thermodynamics #GibbsFreeEnergy #ChemicalReactions #SpontaneousReactions #ReactionCalculations #ChemistryBasics #PhysicalChemistry #ScienceEducation #ChemicalEquilibrium #ReactionQuotient #IndustrialChemistry #Biochemistry #EnergyChanges #ChemistryTips #LearnChemistry About Us: Welcome to Thermodynamics For Everyone! Our channel is dedicated to making the principles of thermodynamics accessible to everyone. We cover essential topics such as the laws of thermodynamics, heat transfer, entropy, energy systems, thermal efficiency, and much more. Whether you're curious about heat engines, the Carnot cycle, or the behavior of gases, our content is designed to help you grasp these concepts in an engaging way.