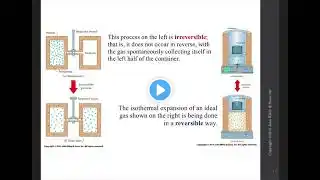
First law of Thermodynamics || Work, Heat & Internal energy || Enthalpy #thermodynamics #chemistry
First law of Thermodynamics || Work, Heat & Internal energy || Enthalpy #thermodynamics #chemistry Thermodynamics-1 • Thermodynamics 1 | B.Sc. 6th semester Chem... telegram I'd -https://t.me/zchemchemistryclasses Thermodynamics-1 : First Law of Thermodynamics : Statement , definition of internal energy and enthalpy. Heat capacity ,heat capacities at constant volume and pressure and their relationship. Joule's law – Joule- Thomson coefficient and inversion temperature . Calculation of w, q, dU & dH for the expansion of ideal gases under isothermal and adiabatic conditions for reversible process. Thermochemistry: Standard state, standard enthalpy of formation – Hess's law of heat summation and its applications. Heat of reaction at constant pressure and at constant volume . Enthalpy of neutralization . Bond dissociation energy and its calculation from thermo-chemical data , temperature dependence of enthalpy. Kirchhoff's equation. Thermodynamics-2 Second Law of Thermodynamics, Need for the law, different statements of the law, Carnot cycle and its efficiency. Carnot theorem. Thermodynamic scale of temperature. Concept of Entropy, Entropy as a state function, entropy as a function of V & T, entropy as a function of P & T, entropy change in physical change, Clausius inequality , entropy as a criteria of spontaneity and equilibrium. Entropy change in ideal gases and mixing of gases. Gibbs and Helmholtz Functions Gibbs function (G) and Helmhotz function (A) as thermodynamic quantities. A & G as criteria for thermodynamic equilibrium and spontaneity, their advantage over entropy change, Variation of G and A with P, V and T. Third Law of Thermodynamics ; Nernst heat theorem , statement and concept of residual entropy. Nernst distribution law – Thermodynamic derivation, applications . #thermodynamic #zchem #rmpssu #mjpru #mgkvp #ccsu #iitjam #cuet #2026 #bsc6thsem #chemistrybysatendrasir #liveclass