Hydroxyl Compounds | Chemical Properties and Reactions | A Level H2 Chem | Making Sense Chem
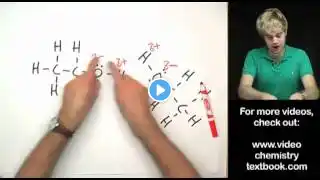
A LEVEL CHEMISTRY!! music by: Zight - Paradise - https://thmatc.co/?l=3B73F710 In today's video, we will be talking about the chemical properties of alcohols. 0:00 Introduction 0:30 Alcohols can be nucleophilic! 2:10 Sn2 mechanism (Williamson ether synthesis) 3:37 Alcohols undergo Nucleophilic Substitution 5:12 Oxidation of an alcohol 6:35 Elimination of an alcohol 8:23 Forming esters from alcohols 14:26 Iodoform test for methyl alcohols 17:39 Chemical test for alcohols Alcohols can be nucleophilic! Lone pair on oxygen atom attacks electron-deficient carbon in R-X Alcohols are weak nucleophiles (due to partial negative charge on O) Before reaction with weak electrophile RX, weak Nu must be converted to a strong Nu first (alcohol to alkoxide) reagent: Na/K(s) conditions: r.t.p Sn2 mechanism (Williamson ether synthesis) Strong nucleophile attacks electron-deficient carbon (nucleophilic substitution) Inversion of stereochemical configuration Transition state is present Chemical reactions of alcohols: Nucleophilic substitution of an alcohol Reagent: PCl₅(s) must be in anhydrous conditions to prevent it from reacting with H₂O to form H₃PO₄ Conditions: r.t.p Products: R-Cl(l) + POCl₃ (l) + HCl(g) White fumes of HCl which is used as distinguishing test for alcohols. Oxidation of 1° alcohol to an aldehyde/carboxylic acid 1° alcohol first oxidises to aldehyde, then carboxylic acid Reagent & conditions: K₂Cr₂O₇/H⁺ heat with distillation (alcohol to aldehyde) Reagent & conditions: K₂Cr₂O₇ or KMNO₄ /H⁺ heat under reflux (alcohol to carboxylic acid) Reagent & conditions: K₂Cr₂O₇ or KMNO₄ /H⁺ heat (aldehyde to carboxylic acid) Oxidation of 2° alcohol Only ketone is formed Reagent & conditions: K₂Cr₂O₇ or KMNO₄/H⁺ heat 3° alcohol CANNOT BE OXIDISED Elimination of an alcohol: Formation of an alkene (removal of a water molecule) Reagent: excess conc. H₂SO₄ / pass alcohol vapour over Al₂O₃ Conditions: heat Removal of the H on the C adjacent to the C directly attached to -OH group Asymmetrical alkenes formed with the most substituted alkene (more stable) being the major product (Saytzeff's rule) Condensation reaction to form an ester: Condensation reaction: two molecules reacting with each other, producing a small molecule in the process. Alcohol + carboxylic acid/ acyl chloride = ester + H2O/HCl Using carboxylic acid (weaker electrophile): Reversible reaction because water formed as the side product reacts with the ester again Reagents & condition: carboxylic acid, few drops of conc. H₂SO₄ with heat Nucleophilic oxygen in -OH attack electrophilic carbon in the -COOH group Incomplete reaction with a lower yield of ester (hence, dehydrating agent H2SO4 required to push POE to the right to increase yield, recall Le Chatelier's Principle) Using acyl chloride (stronger electrophile): Reagent & condition: acyl chloride at r.t.p Irreversible reaction Complete reaction with a higher yield of ester Oxidation of methyl alcohol Carbon MUST be attached to -OH, -CH₃ Reagent & condition: I₂ (aq) and NaOH (aq), WARM (prevent CHI₃ from breaking down) Pale yellow ppt/crystals of CHI₃ with antiseptic smell Step-down reaction due to decrease in 1 C in the product Chemical test for alcohols 1) Na metal Effervescence of colourless and odourless gas that extinguishes burning splint with a 'pop' sound 2)PCl₅ or SOCl₂ White fumes of HCl is seen 3) KMNO₄/K₂Cr₂O₇ with H₂SO₄ Purple KMNO₄ decolourises/orange K₂Cr₂O₇ turns green 3° alcohols are not oxidised Looking for free chemistry resources? Vist our website at: https://www.makingsense-sg.com/free-t... to view other learning resources (content videos + free resources). Follow us on: Youtube - Making Sense Tuition Centre Tik tok - @makingsensechemistry Instagram - @makingsensechemistry Facebook - Making Sense, Singapore's Leading Chemistry Tuition The Best Chemistry tuition! #indigoeducationgroup #bestchemistrytuition #makingsensechemistry #learningisfun #videolessons #educationalvideos #easyandfunlearning #alcohols #chemicalproperties #chemicalreactions