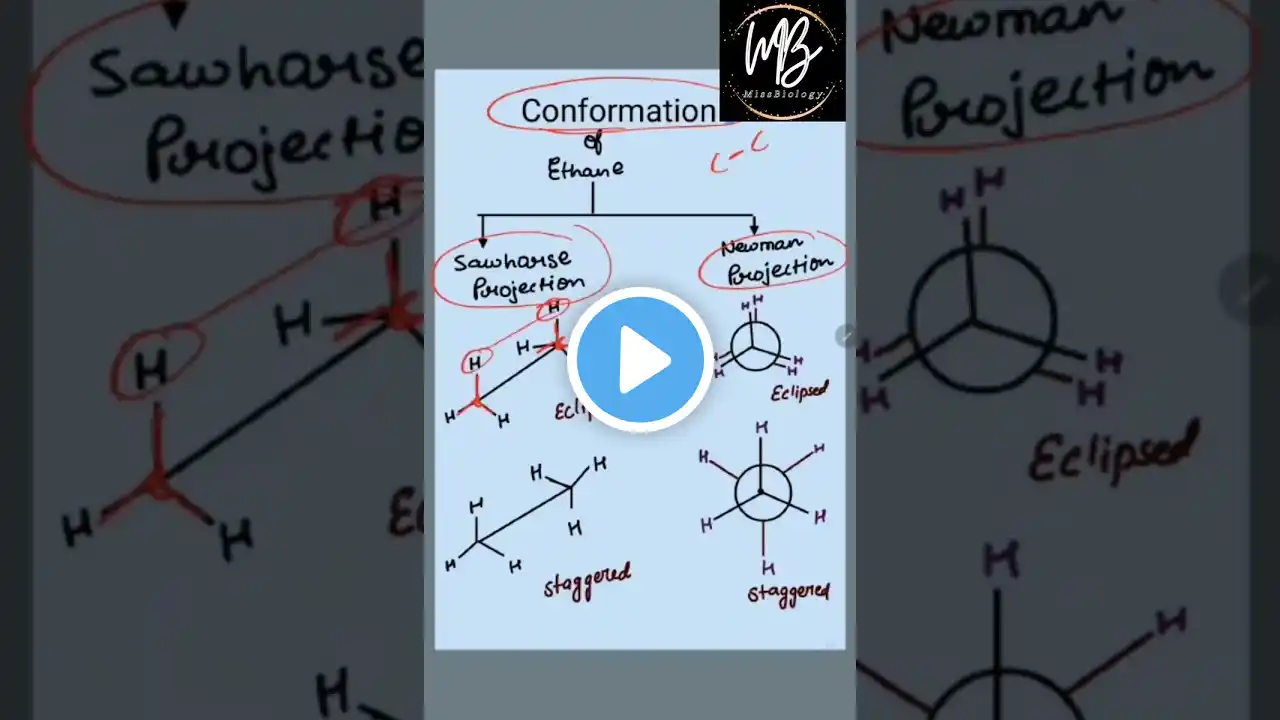
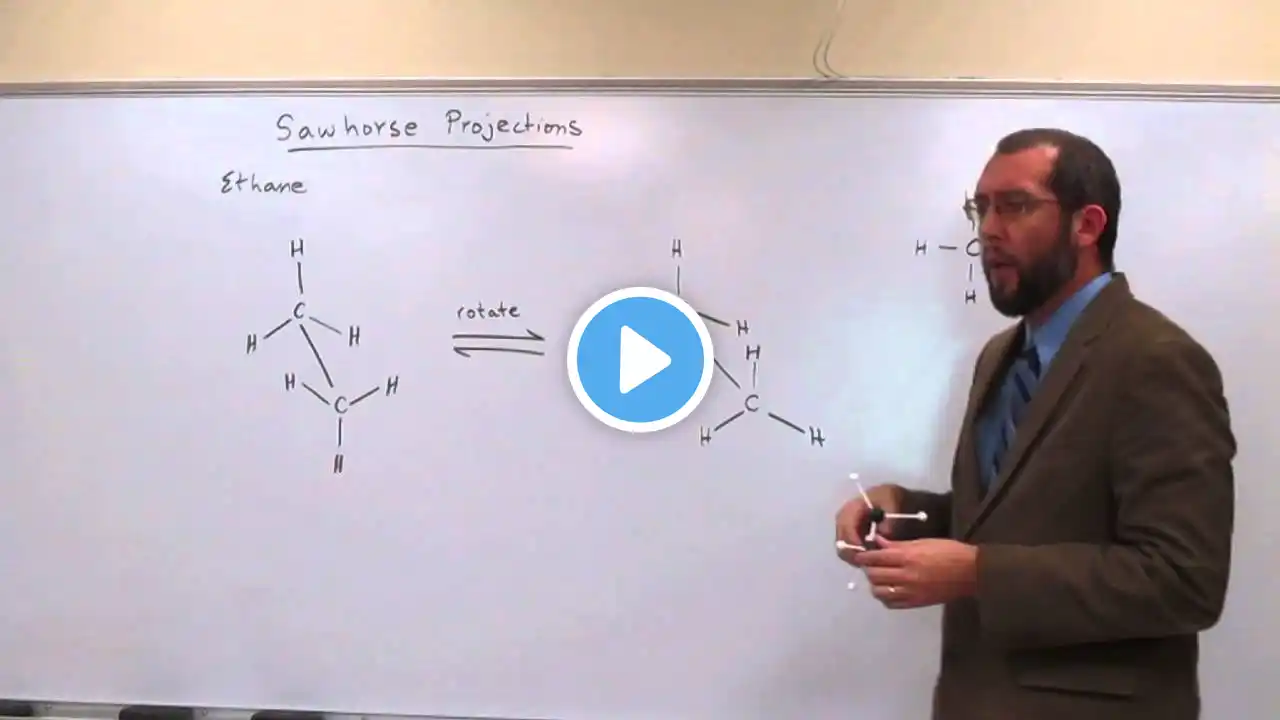
SAW-HORSE, NEWMANN, WEDGE DASH, FISCHER PROJECTIONS (ISOMERISM) , CONFORMATIONS OF ETHANE, BUTANE
Dive into the fascinating world of isomerism in organic chemistry! This video breaks down the concept of isomers—molecules with the same chemical formula but different atomic arrangements. We'll explore why these structural differences lead to unique properties and cover the two main categories: What you'll learn: Definition of Isomerism: Understand the core principle that shape affects function in chemistry. Structural (Constitutional) Isomers: Learn about compounds with different connectivities between atoms. Chain Isomerism: Differences in the carbon backbone structure. Position Isomerism: Different positions of a functional group or substituent. Functional Group Isomerism: Same molecular formula, but different functional groups. Stereoisomers: Explore compounds with the same connectivity but different 3D spatial arrangements. Geometrical Isomerism (Cis/Trans or E/Z): Non-rotation around double bonds leading to different spatial orientations. Optical Isomerism: The concept of chiral centers and non-superimposable mirror images (enantiomers)