How Do You Calculate Enthalpy Change Using Heats Of Formation? - Chemistry For Everyone
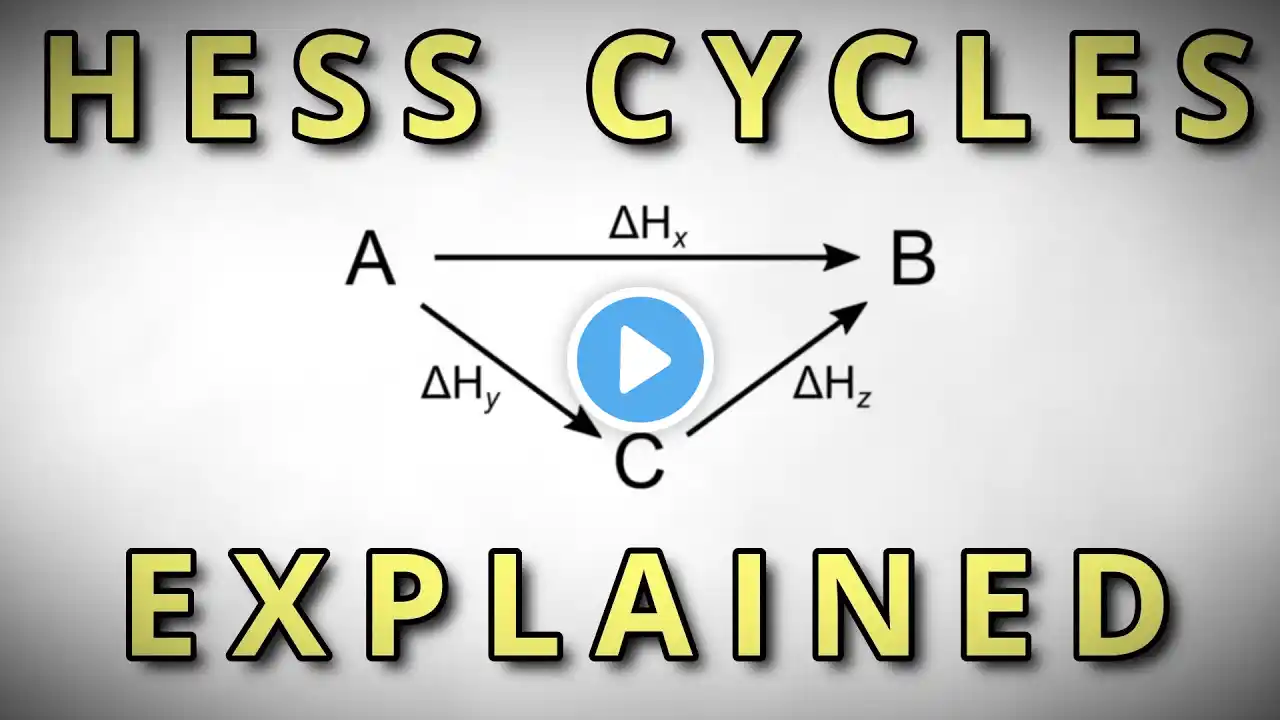
How Do You Calculate Enthalpy Change Using Heats Of Formation? Have you ever wondered how scientists determine the energy involved in a chemical reaction? In this informative video, we'll explain the process of calculating enthalpy change using heats of formation. We'll start by discussing the importance of balanced chemical equations and how coefficients represent the number of moles involved in reactions. You'll learn how to find standard heats of formation from tables and why elements in their most stable states have a heat of formation of zero. We'll guide you through multiplying heats of formation by the number of moles for each substance, both reactants and products, to set up your calculation correctly. Next, we'll show you how to add up heats of formation for all products and reactants separately, and then find the difference to determine the overall energy change of the reaction. If the result is negative, it indicates an exothermic process, while a positive value signifies an endothermic one. This method is based on Hess's Law, which states that the total enthalpy change depends only on the initial and final states of the reaction, regardless of the pathway taken. Understanding this concept makes it easier to analyze reactions like fuel combustion or synthesis of complex compounds. Join us for this clear explanation, and subscribe to our channel for more insights into chemistry and thermochemistry. ⬇️ Subscribe to our channel for more valuable insights. 🔗Subscribe: https://www.youtube.com/@Chemistry-Fo... #Chemistry #Enthalpy #HeatsOfFormation #Thermochemistry #EnergyChanges #ChemicalReactions #HesssLaw #ChemistryTips #ScienceEducation #ChemicalEnergy #ReactionCalculations #ScienceExplained #ChemistryForBeginners #EnergyInReactions #LearnChemistry About Us: Welcome to Chemistry For Everyone, your go-to destination for exploring the fascinating world of chemistry and materials science! Our channel is dedicated to making complex concepts accessible and enjoyable for everyone, from curious beginners to seasoned enthusiasts.