O Level iGCSE | Live Class 41 | Moles and Stoichiometry | Empirical Formula | +92 323 509 4443
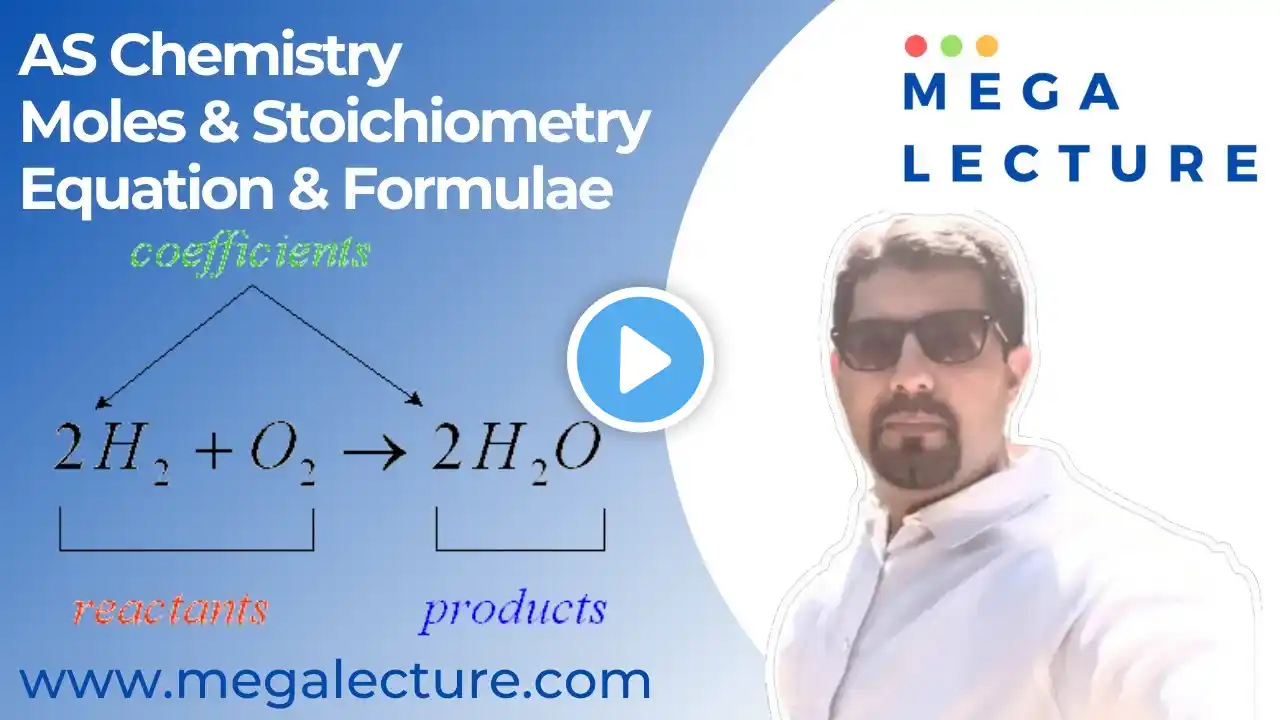
To join our Free Trial Lesson, Join our Whatsapp Community Now! https://chat.whatsapp.com/CWSRaMi4i3w... or msg at +92 323 509 4443 To purchases recorded courses with notes https://megalecture.com/courses/ Welcome to Live Class 41 in our O Level iGCSE Chemistry series! In this session, we’re covering Moles and Stoichiometry with a focus on calculating the Empirical Formula—a fundamental concept in understanding chemical composition. Topics Covered: Understanding Empirical Formula The empirical formula represents the simplest whole-number ratio of atoms in a compound. We’ll explain how it differs from the molecular formula and why it’s useful for analyzing chemical substances. Steps to Calculate Empirical Formula Learn the step-by-step method to calculate the empirical formula from mass or percentage composition. We’ll work through examples to illustrate each step, making the process straightforward. Practice with Real-Life Examples This class includes practice questions where we’ll determine the empirical formulas of various compounds based on given data. These examples will help you apply the concepts effectively. Exam Practice and Tips Get tips for tackling empirical formula questions in exams, including common pitfalls to avoid and strategies to ensure accuracy in your calculations. For any additional help or questions, contact us on WhatsApp at +92 323 509 4443. Join this session to build a solid understanding of empirical formulas and strengthen your skills in moles and stoichiometry for O Level Chemistry exams! Don’t forget to like, share, and subscribe for more iGCSE Chemistry lessons. Enable notifications to stay updated on our latest content! #OLevelChemistry #IGCSE #MolesAndStoichiometry #EmpiricalFormula #ExamPreparation #ChemistryClasses #WhatsAppSupport