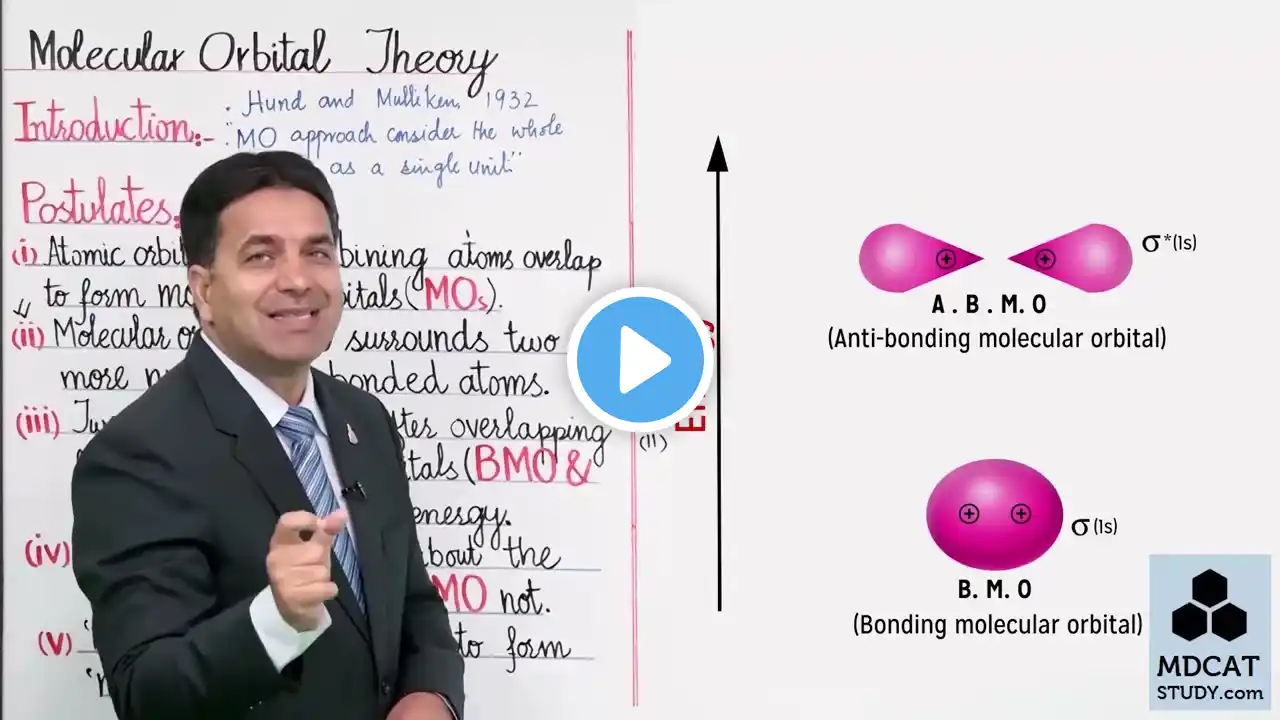
FSC 1st year chemistry new book chap 3 topic molecular orbital diagram of diatomic molecules
In this lecture of FSc 1st Year Chemistry (New Book 2025), Chapter 3: Chemical Bonding, we will study the very important topic of Molecular Orbital Theory (MOT) and learn how to draw Molecular Orbital (MO) diagrams of some diatomic molecules. 🔹 First, we will revise the basic concept of Molecular Orbital Theory and understand how atomic orbitals combine to form bonding and antibonding molecular orbitals. 🔹 Then, we will discuss the energy order of molecular orbitals for lighter elements (H₂ to N₂) and heavier elements (O₂ to Ne₂). 🔹 Step by step, we will draw MO diagrams for important diatomic molecules such as: H₂ (Hydrogen molecule) He₂ (Helium molecule) Li₂ (Lithium molecule) B₂ (Boron molecule) C₂ (Carbon molecule) N₂ (Nitrogen molecule) O₂ (Oxygen molecule) F₂ (Fluorine molecule) Ne₂ (Neon molecule) 🔹 We will also calculate the bond order, magnetic behavior (paramagnetism/diamagnetism), and stability of each molecule based on its molecular orbital diagram. By the end of this lecture, you will clearly understand: ✔ How to fill electrons in molecular orbitals using Aufbau principle, Pauli’s rule, and Hund’s rule ✔ Difference between energy order of MOs for lighter and heavier molecules ✔ Why some diatomic molecules exist (like N₂, O₂) while some do not (like He₂, Ne₂) ✔ Explanation of bond order formula and its application ✔ Why O₂ is paramagnetic (has unpaired electrons) This topic is highly important for board exams, conceptual MCQs, and numerical-based questions in FSc Part 1 Chemistry. ✨ Stay connected with Science Teaching Zone for more lectures on Chemistry, Physics, and Biology (FSc Part 1) according to the Punjab Board New Syllabus 2025. --- 🔖 Hashtags #ScienceTeachingZone #FScChemistry #Class11Chemistry #ChemicalBonding #MolecularOrbitalTheory #MODiagram #DiatomicMolecules #BondOrder #ParamagneticOxygen #SigmaAndPiBonds #PunjabBoard2025 #FScPart1 #NewSyllabus2025