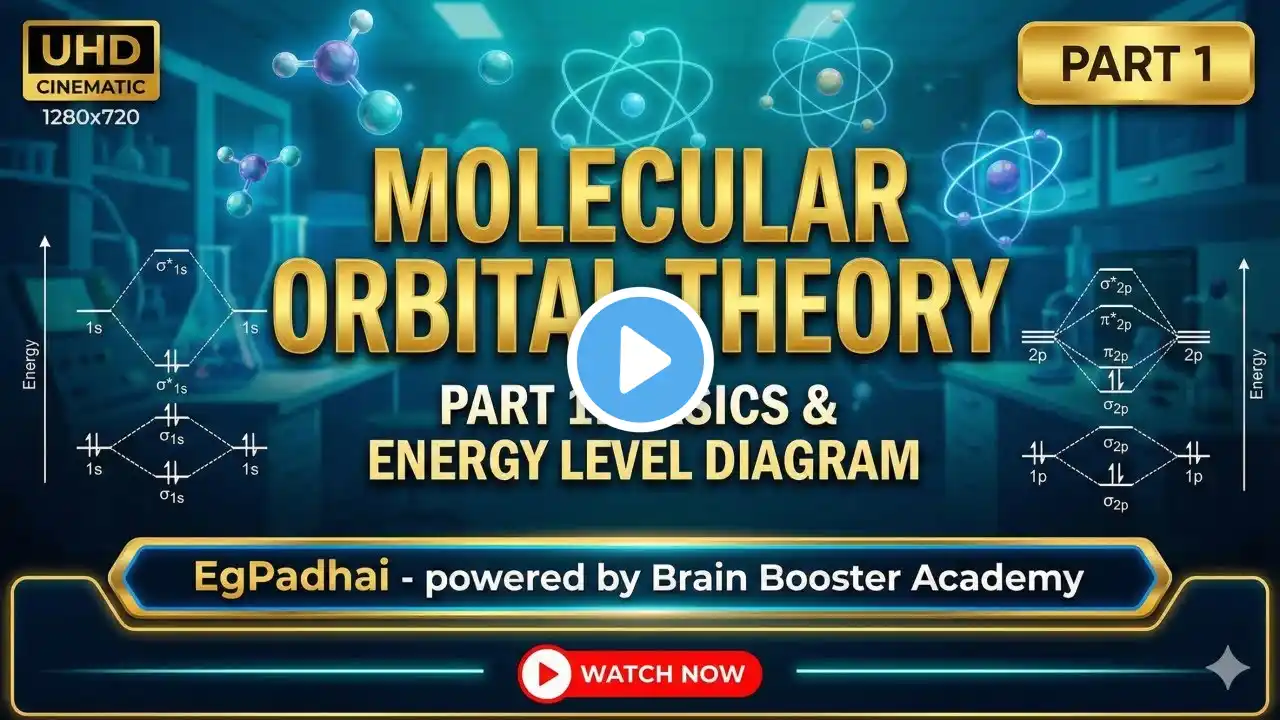
Molecular Orbital Theory (MOT) | Chemical Bonding Class 11|Full Concept & Tricks|JEE/NEET|Ritesh Sir
Welcome to Brain Booster Academy! In this video, Ritesh Sir gives a complete and detailed breakdown of the Molecular Orbital Theory (MOT). This is one of the most important topics of the Chemical Bonding and Molecular Structure chapter for Class 11, JEE Mains, Advanced, and NEET aspirants.अगर आपको VBT के बाद MOT समझने में दिक्कत होती है, या Bond Order निकालने में कन्फ्यूजन है, तो यह वीडियो आपके लिए है। हमने बहुत ही आसान भाषा (Hinglish) में Examples के साथ सब कुछ समझाया है।🌟 Key Highlights of this Lecture:✅ Why did Valence Bond Theory (VBT) fail? (Limitations of VBT)✅ History and Introduction to Molecular Orbital Theory (MOT).✅ Postulates of MOT (BMO vs ABMO, LCAO).✅ Rules for filling electrons in Molecular Orbitals (Aufbau, Pauli, Hund's Rule).✅ Detailed MO Diagrams of Hydrogen ($H_2$) and Oxygen ($O_2$).✅ Super Trick: How to find Bond Order, Stability, and Magnetic Nature (Paramagnetic vs Diamagnetic).यह वीडियो उन सभी छात्रों के लिए है जो क्वालिटी एजुकेशन फ्री में प्राप्त करना चाहते हैं।⏱️ Timestamps (Chapters):(वीडियो अपलोड करने के बाद सही समय यहाँ डालें, यह SEO के लिए बहुत जरूरी है)00:00 - Introduction to MOT & Brain Booster Academy02:15 - Why was MOT needed? (Failure of VBT)05:30 - Core Concept & Postulates of MOT12:45 - What are BMO and ABMO?18:20 - Energy Level Diagram & Filling Rules25:10 - MO Diagram of H2 Molecule (Example 1)32:40 - MO Diagram of O2 Molecule (Example 2)40:15 - Why Oxygen (O2) is Paramagnetic?45:00 - Bond Order Formula & Stability Tricks52:30 - Conclusion & Summary📚 Related Playlists:Chemical Bonding Full Chapter: [Link to your playlist here]Class 11 Chemistry Complete Course: [Link here]Connect with Brain Booster Academy:Subscribe for more Free Educational Content!👍 Like this video if you found it helpful.🔔 Turn on notifications to never miss an update.📢 Share this video with your friends and classmates!#ChemicalBonding #MolecularOrbitalTheory #RiteshSir #BrainBoosterAcademy #FreeEducation


















