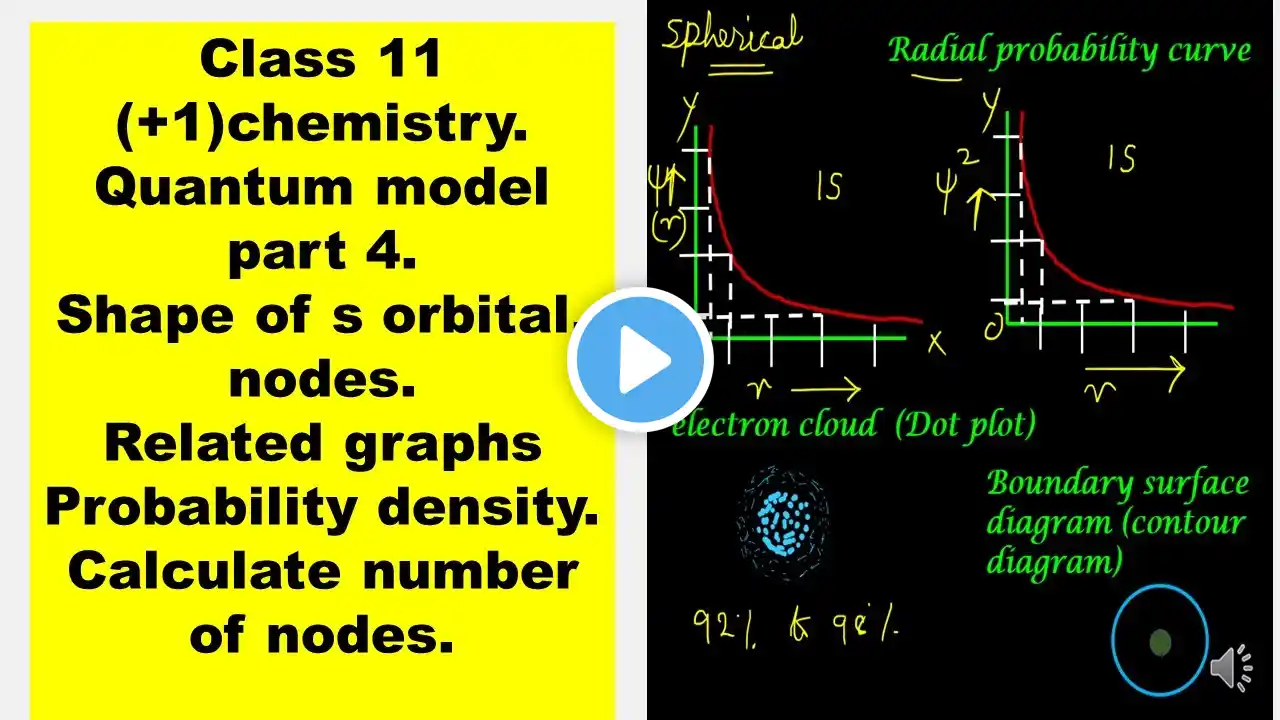
shape of s orbital/nodes/probability density/quantum model/class11 chemistry/structure of atom
shape of s orbital/nodes/probability density/quantum model/class11 chemistry/structure of atom /guidancee education video 2.16 #shapeofsorbital#nodes#probabilitydensity#quantummodel #class11chemistry#structureofatom#guidanceeeducation shape of s atomic orbital is spherical wave function Vs distance curve for 1 s and 2s is presented and explained. variation of probability density with distance is presented and explained. the dot plot or electron cloud and the boundary surface diagram are also explained , expression for wave function and probility density are given node is the region where the probability density is zero. number of nodes for an orbital = n - l - 1 question 1.calculate the number of nodes for 4s orbital 2. what is the need to calculate probability density? 3. plot the probability density curve for 1 s and 2 s orbitals and also draw the corresponding electron cloud picture. you may post your answers for q1 and q2 in the comment box. subscribe. thanks take care. keep working good luck