Double Displacement Reaction | Class 10 Science | CBSE | Explained with Easy Examples in Hindi
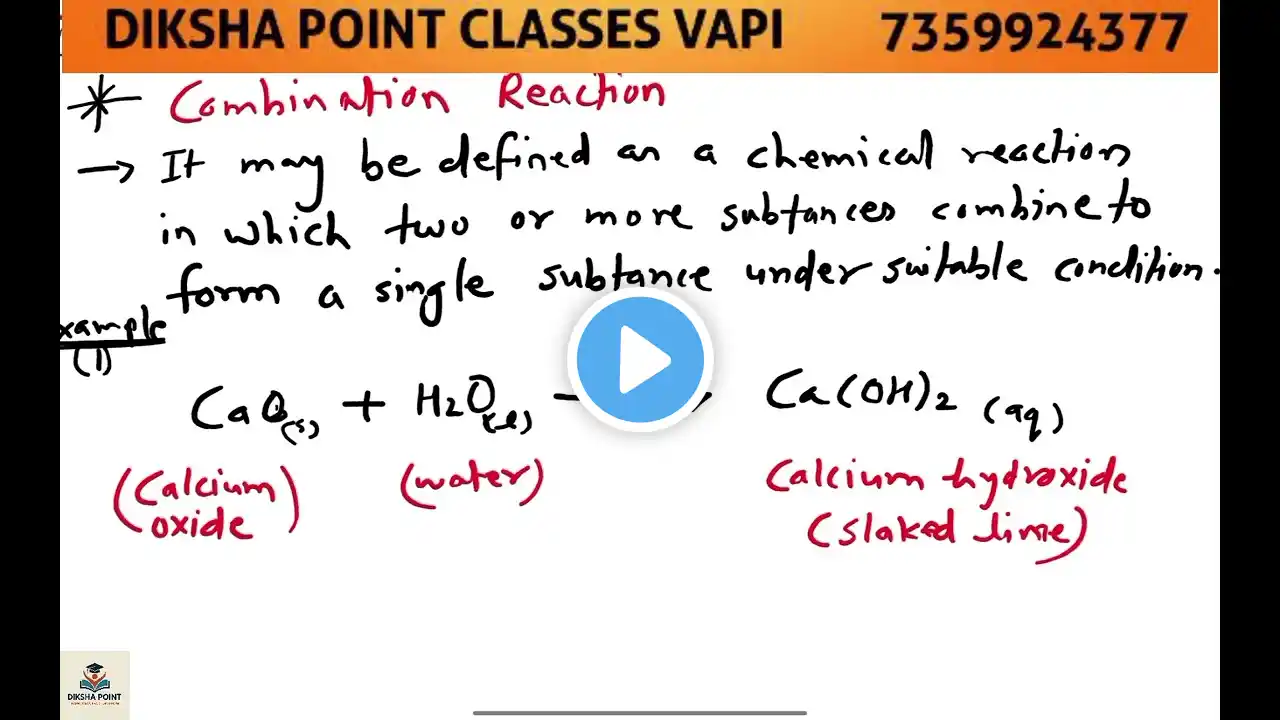
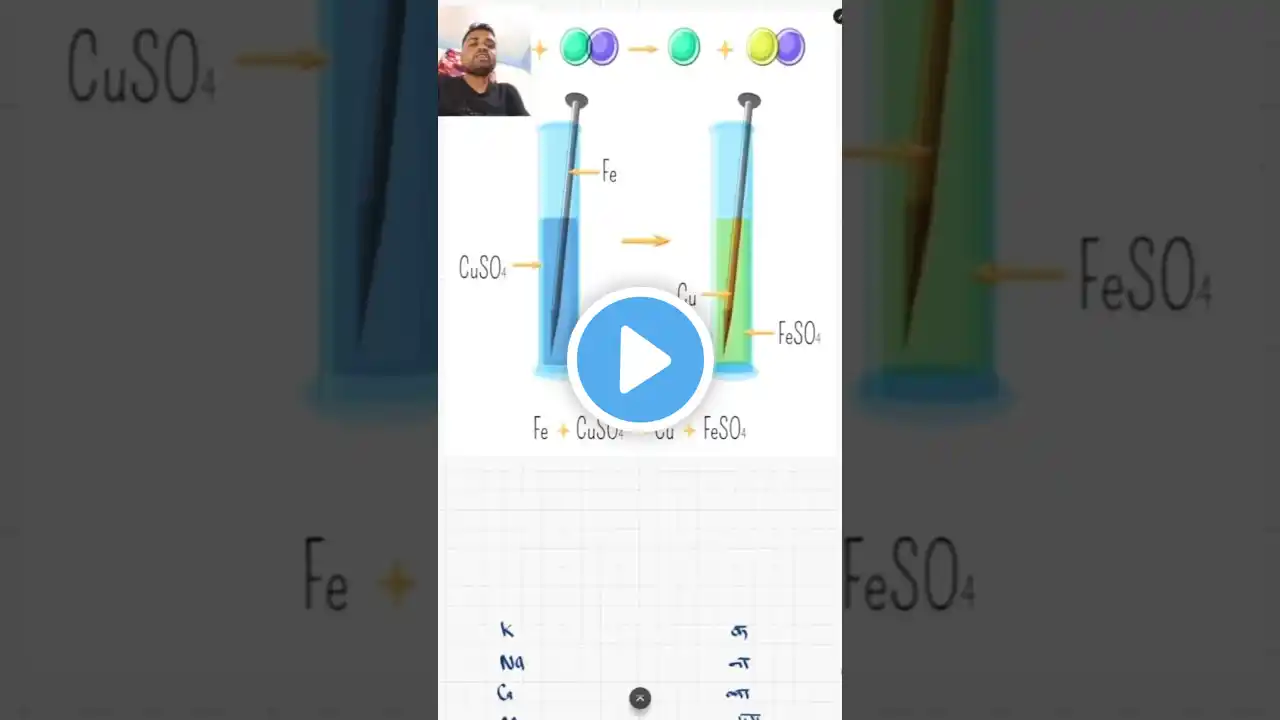
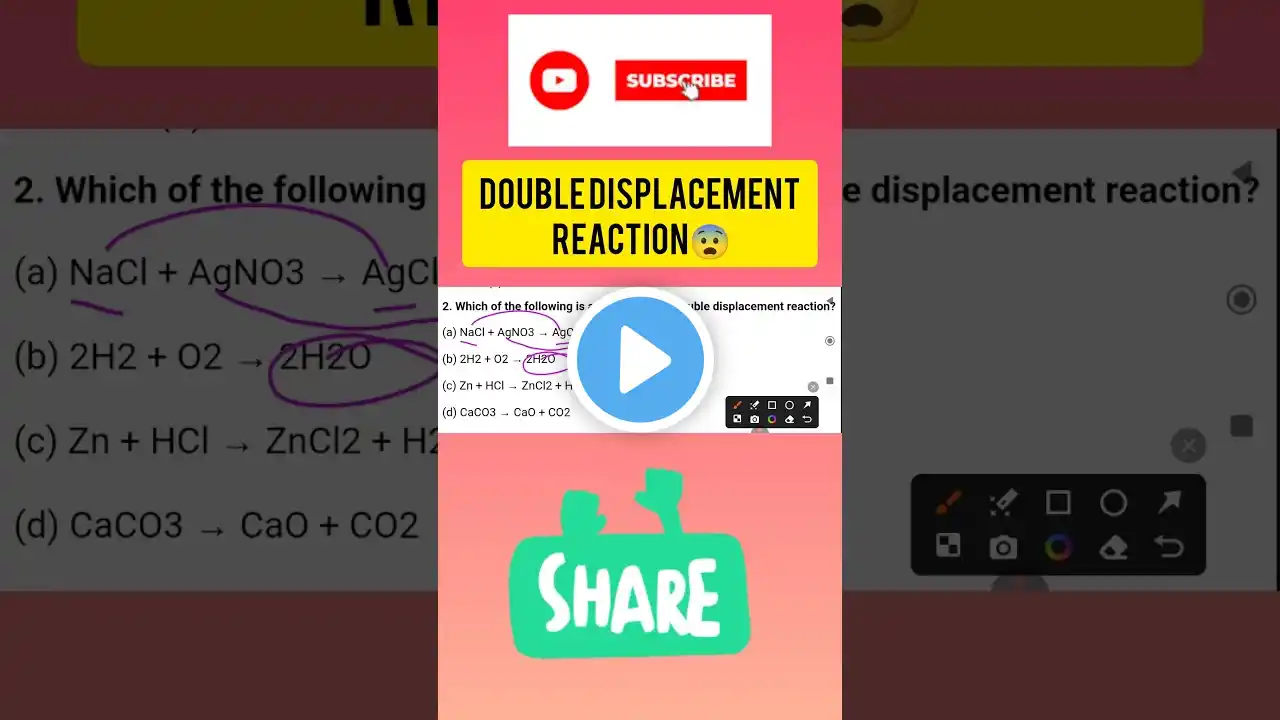
Welcome to our science learning series! In this video, we are going to dive deep into the Double Displacement Reaction, an important type of chemical reaction covered in Class 10 CBSE Science under the chapter Chemical Reactions and Equations. This reaction is not only vital for exam preparation but also forms the foundation for understanding complex chemical processes in real life. So, what exactly is a double displacement reaction? In simple terms, it is a type of chemical reaction where the positive ions (cations) and negative ions (anions) of two different compounds switch places to form two new compounds. This reaction typically occurs in aqueous solutions and often results in the formation of a precipitate, gas, or water. This video is perfect for CBSE students who want to master this concept quickly and efficiently. We explain the topic in Hindi, keeping it simple, clear, and exam-focused. With examples like: Reaction between Barium Chloride and Sodium Sulphate, Reaction between Hydrochloric Acid and Sodium Hydroxide, you’ll see how the ions exchange and form new compounds. We also cover: Definition of Double Displacement Reaction Key characteristics and conditions Real-life applications Common mistakes to avoid in exams Practice questions with answers All of this is presented in a smartboard-style video, using black background and bold white text for clear visibility and focus—especially helpful for online learning and YouTube video lectures. This lesson aligns with NCERT curriculum and follows the R.S. Aggarwal approach many schools adopt for reference. Whether you're studying for school exams, board exams, or just want to understand the concept thoroughly, this video is for you! Don’t forget to like, comment, and subscribe for more such content. Tap the bell icon to stay updated with our latest science and math lessons. Happy learning! Double displacement reaction Double displacement reaction class 10 CBSE class 10 chemical reactions What is double displacement reaction Examples of double displacement reaction Class 10 science explanation Chemical reaction types Double displacement reaction in Hindi Science chapter chemical reactions NCERT class 10 science Reactions forming precipitate Reaction forming gas Neutralisation reaction Displacement vs double displacement Ionic reactions class 10 Easy explanation science Smartboard science lecture Class 10 board exam preparation Hindi science explanation Double displacement equation Barium chloride reaction Sodium sulphate example Acid base reaction Hydrochloric acid with base NaOH reaction Water formation in reaction Color change in reaction CBSE board science Class 9 double displacement Reactivity of ions Practical example class 10 CBSE class 10 science chapter 1 Hindi medium science class 10 Science concept clarification Online science tuition Blackboard science teaching Double displacement animation Science YouTube lesson Classroom science content Chemical bonding basics Reaction mechanism class 10 #DoubleDisplacementReaction #Class10Science #ChemicalReactions #ScienceInHindi #CBSEClass10 #BoardExamPreparation #ScienceLecture #HindiScienceVideo #SmartboardTeaching #ScienceWithExamples #DoubleDisplacementClass10 #ReactionTypes #CBSE2025 #NCERTScience #OnlineLearning #ScienceShorts #DoubleDisplacementExplained #EasyScienceLecture #ChemicalEquations #ScienceMadeEasy #DoubleDisplacementReactionInHindi #ChemistryClass10 #AcidBaseReaction #PrecipitationReaction #ScienceTutoring #Class10CBSE #ScienceConcepts #SodiumHydroxide #BariumChloride #ScienceAnimation #BoardExamScience #ReactionExplanation #YouTubeScienceClass #HindiMediumEducation #ExamFocusedLecture #CBSEPreparation #CBSEScienceChapter1 #ScienceForKids #EduContentHindi #NCERTBasedLearning