Reformatsky Reaction
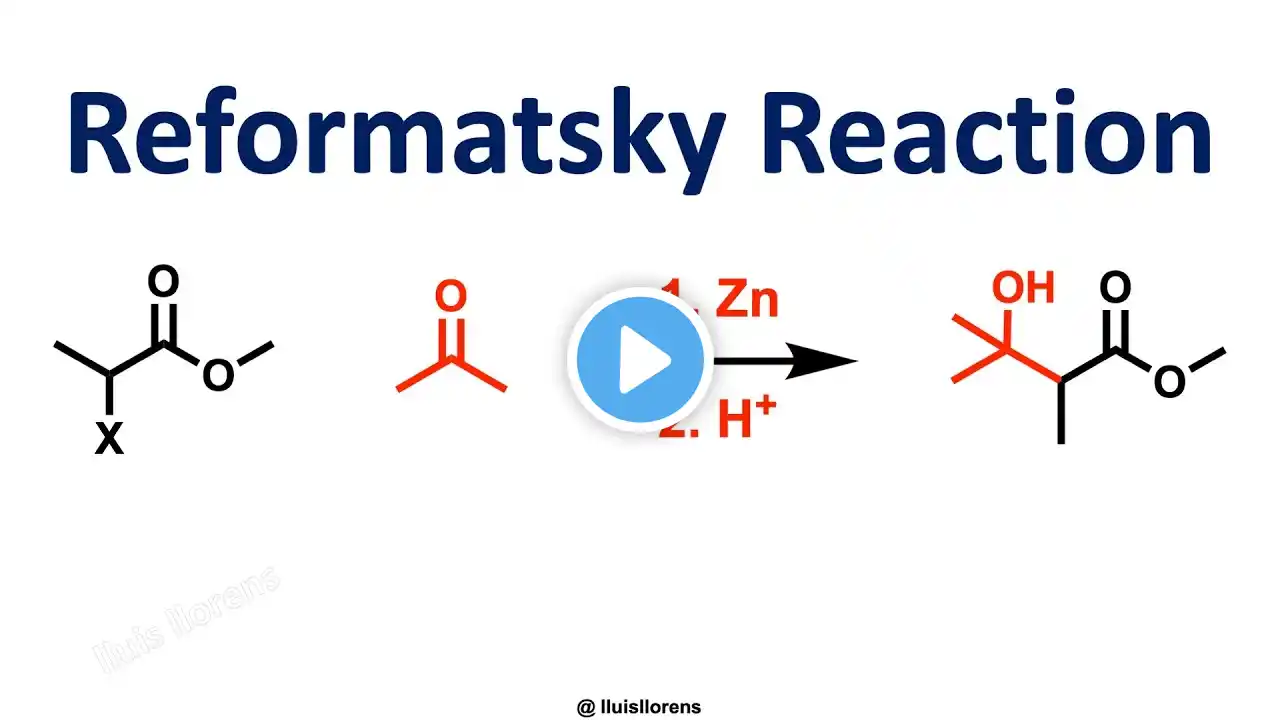
The Reformatsky Reaction is the zinc-induced reaction between alpha-halo esters and aldehydes or ketones. Also, a metal-induced reaction of alpha-carbonyl halides with a wide range of electrophiles. General features: 1. The Reformatsky reaction is a good alternative to the classical aldol reaction. 2. The reaction works with highly substituted ketone substrates. 3. The organozinc reagent, or 'Reformatsky enolate,' is prepared by treating an alpha-halo ester with zinc dust. 4. The ester enolate can be formed in the presence of highly enolizable aldehyde and ketone functionalities. 5. The Reformatsky reaction is suitable for intramolecular reactions. Reaction mechanism: 1. Oxidative addition. The activated zinc metal inserts into the carbon-halogen bond. 2. The resulting compound dimerizes and rearranges to form two zinc enolates. 3. Reaction of the zinc enolate with the carbonyl compound (aldol reaction) through a six-membered chair-like transition state. 4. Acid workup removes zinc to yield the corresponding beta-hydroxy ester. Find the mechanism here: https://www.pinterest.es/pin/67166973.... Pinterest: https://www.pinterest.es/pin/67166973... References: https://nrochemistry.com/reformatsky-... Seminal publication: Reformatsky, S. Ber. 1887, 20, 1210-1211. https://doi.org/10.1002/cber.18870200... Related videos: Aldol reaction: • Aldol Addition and Condensation Claisen condensation: • Claisen Condensation


















