Bromonium Overview
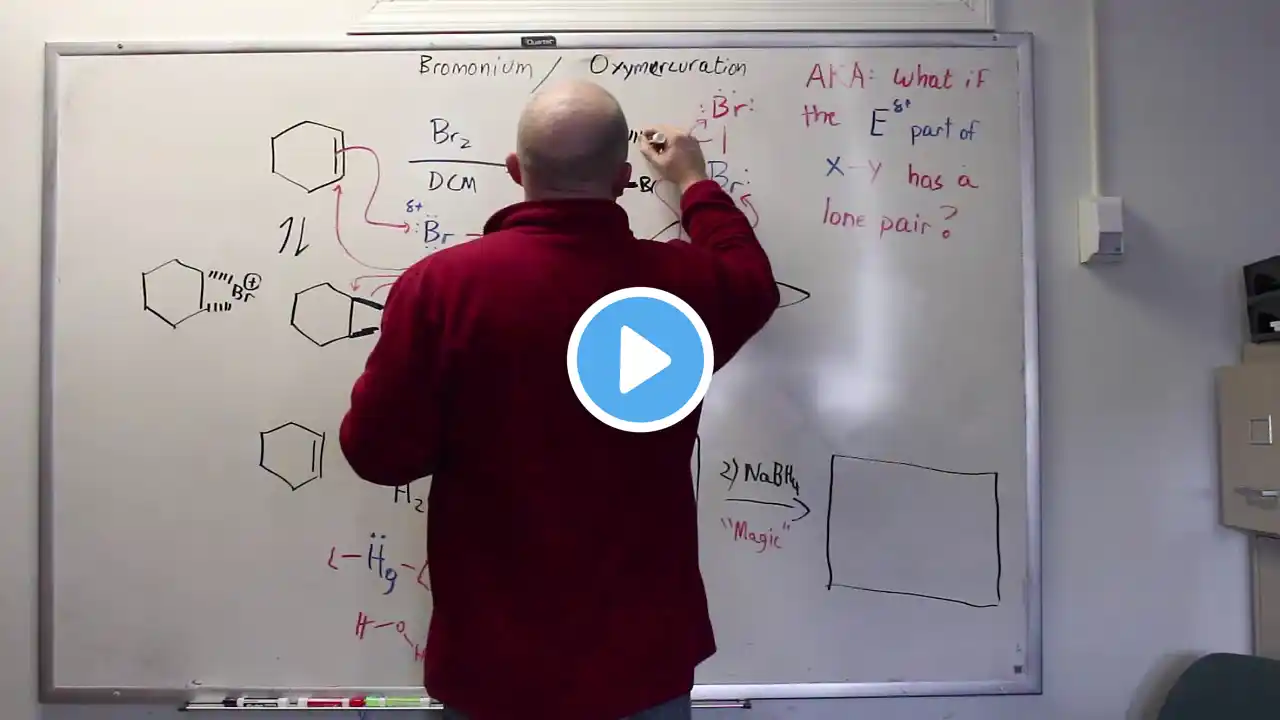
Chem 240 Videos - Video 51 Bromonium ions form because when a Bromine serves as an electrophile, it has a pair of electrons on it. That will serve to stabilize the nearby carbocation (that otherwise would form) - so... the Bromine sits roughly on top of where the former double bond sat, making a full bond to the less substituted side, and a partial bond on the more substituted side. In our Lewis model, we draw both bonds the same, but mentally think of the bromine shaded over to the LESS substituted side. By analogy, the Oxymercuration first step works the same way.