MEC1001 Part 8 b
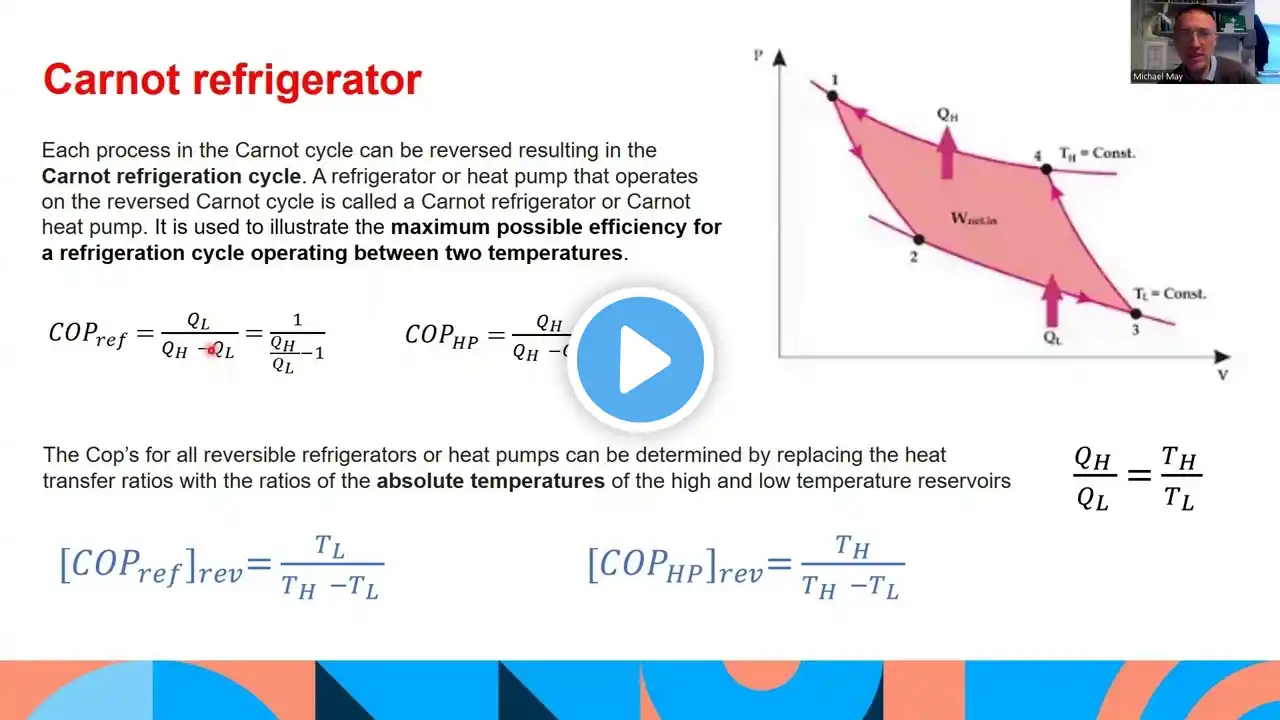
Summary The video delves into the principles of the Second Law of Thermodynamics and its implications on heat engines, refrigeration, and heat pumps. The Second Law states that while energy cannot be created or destroyed (First Law), it does dictate the directionality of energy transfer, inherently favoring spontaneity from high to low temperature states. The lecture explains how energy flows from higher to lower “quality”, using the example of mechanical systems that fail to revert to their original form without additional work input. Essential concepts, including thermal energy reservoirs, heat engines, and the Carnot cycle, are introduced to explain these dynamics. A thermal energy reservoir is highlighted as a hypothetical body that can absorb or provide heat without significant temperature change, illustrated with large bodies of water in power generation. Heat engines are defined as systems that convert thermal energy into mechanical work amidst inevitable energy losses, reinforcing that achieving 100% efficiency is impossible due to the Second Law. The Carnot cycle, a theoretical model of a heat engine, aims to establish the maximum potential efficiency possible, expressed through temperature ratios. The concept of reversible processes is introduced, where energy transformations occur without loss, contrasting with irreversible processes where energy is dissipated through friction or unrestrained expansion. The lecture concludes by discussing perpetual motion machines, emphasizing their impossibility under the laws of thermodynamics, particularly regarding the violations of energy conservation and efficiency. Highlights 🔥 Second Law of Thermodynamics: Dictates the direction of energy transfer, emphasizing high to low temperature flow. ⚙️ Heat Engines: Defined as devices converting heat into mechanical work, characterized by energy losses. 💧 Thermal Energy Reservoirs: Hypothetical bodies that can absorb or provide heat without temperature change. 🔄 Carnot Cycle: A theoretical model for determining the maximum efficiency of heat engines, significant for thermodynamics. ⚖️ Reversible Processes: Idealized scenarios allowing no energy loss, maximizing work output and efficiency. 🚫 Limits of Efficiency: Emphasizes that 100% efficiency is impossible under the Second Law. ♻️ Perpetual Motion Machines: Highlighted as impossible constructs violating fundamental laws of thermodynamics. Key Insights 🌀 Energy Transfer Directionality: The Second Law of Thermodynamics underscores that energy spontaneously moves from regions of high energy (high temperature) to low energy (low temperature). This establishes a universal principle that governs thermal dynamics, where systems are prevented from naturally reverting their states without work input. ✨ Practicality of Heat Engines: The design of heat engines, which include systems like steam turbines and refrigerators, relies on controlled energy transfers where heat is absorbed at high temperatures and rejected at low temperatures. This cycle is inherently inefficient due to losses, reinforcing the idea that real-world applications must grapple with inefficiencies inherent in thermodynamic processes. 🌊 Understanding Thermal Reservoirs: The concept of thermal energy reservoirs helps us visualize large systems that can provide or absorb heat without a noticeable change in temperature, such as oceans or large lakes. This principle is crucial in understanding how power plants operate without significantly affecting their heat sources. 🏭 Carnot Cycle Efficiency: By establishing the Carnot cycle as a benchmark for efficiency, the video underscores the significance of temperature differences in optimizing engine performance. The efficiency (1 - TL/TH) is a pivotal formula, highlighting how higher temperature differences yield better performance in thermodynamic systems. 📈 Reversible vs. Irreversible Processes: Real-world systems typically operate under irreversible conditions due to friction and other dissipative effects. By comparing reversible and irreversible processes, we can better understand energy efficiency and the theoretical limits of work production in heat engines. ⚠️ Impossibility of Perpetual Motion: The lecture clarifies the futility of creating perpetual motion machines, as they defy the First and Second Laws of Thermodynamics. Attempts to create such systems highlight fundamental misunderstandings of energy conservation and transformation limits. 🔬 Innovation within Constraints: Recognizing the limitations imposed by thermodynamic laws fosters innovations that seek to optimize energy use within existing frameworks, urging engineers and scientists to improve efficiency creatively rather than contrarily approaching unrealistic expectations like 100% efficiency.