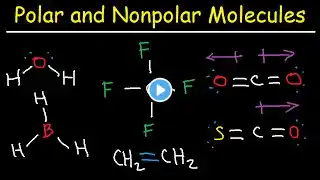
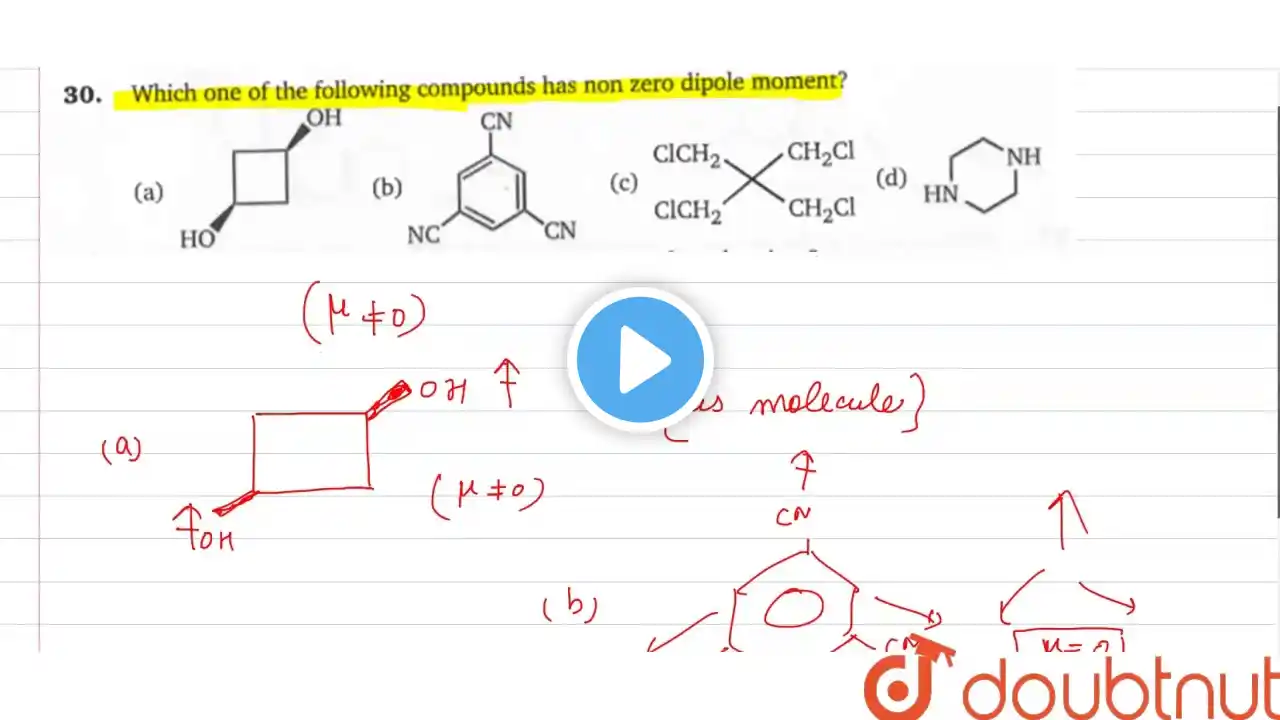
Which compound below could have a zero dipole moment?
Which compound below could have a zero dipole moment? A. CuCl2F2 (tetrahedral) B. PtCl2F2 (square planar) C. SCl2F2 (see-saw) D. CCl2F2 (tetrahedral) 🔴 Subscribe for more Chemistry content: / @chemistrymvp 🧪 Website - Visit our website at www.chemistrymvp.com for homework help. 🙋♂️ Have a question? Ask it in the comment section and I will be sure to answer it ASAP. 🤩 If you like the content be sure to give this video a like 👍 and a comment 💬. Both of those things helps bring you more chemistry videos. 🎥 Watch our most recent video: / @chemistrymvp 📸 Follow us on instagram / chemistrymvp to see different experiments 🎈🔥 and keep up with all the AP Chem news 📰.