Kinetic Molecular Theory (KMT), Ideal Gas Concepts, Kinetic Energy, Molecular Speed, and Effusion
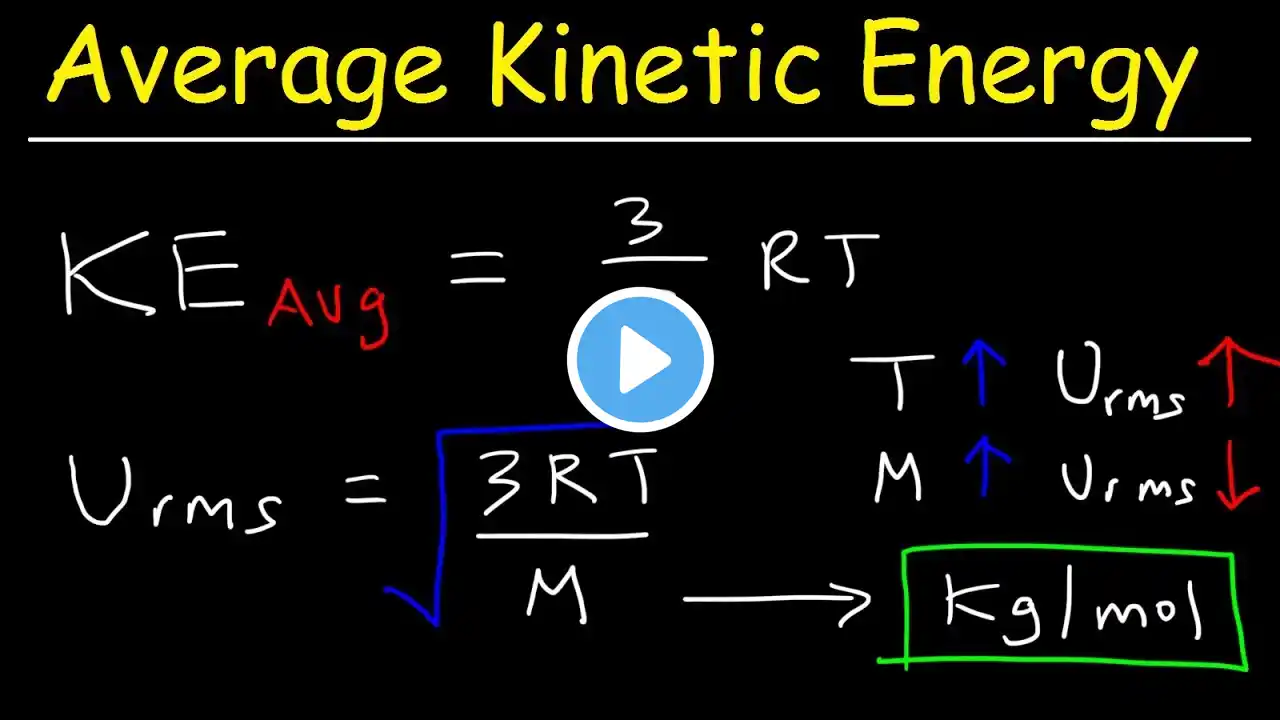
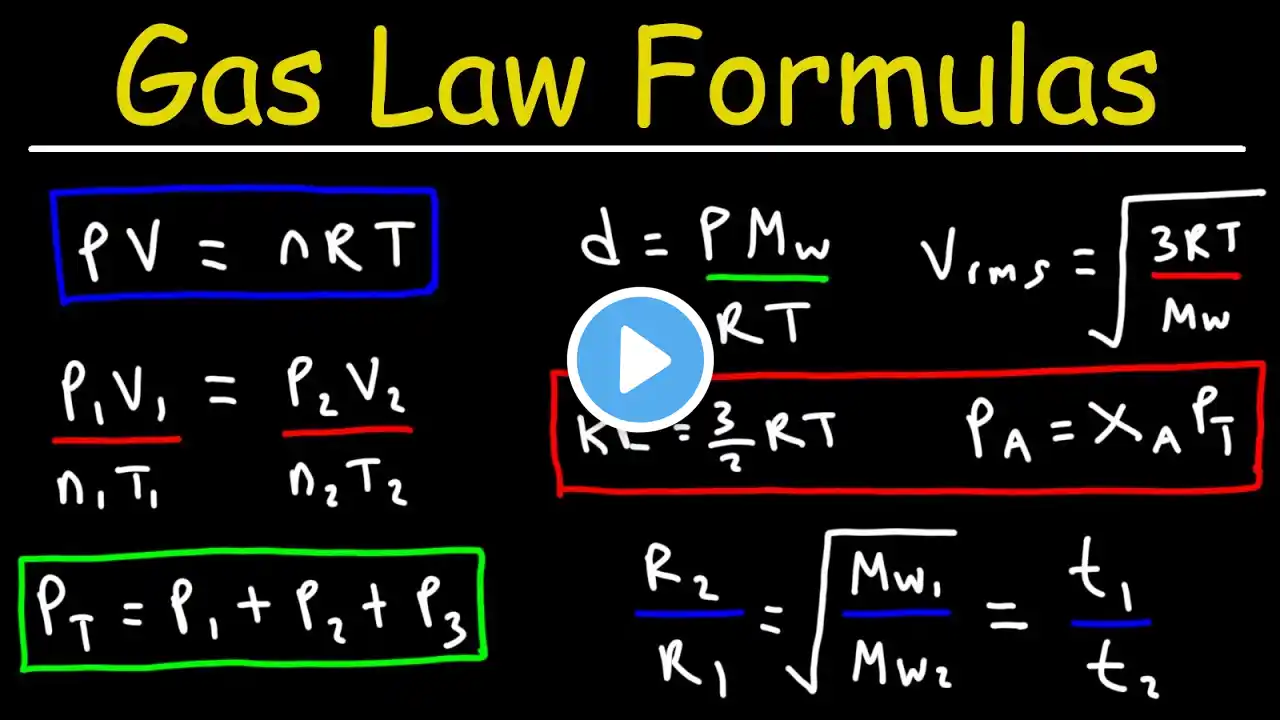
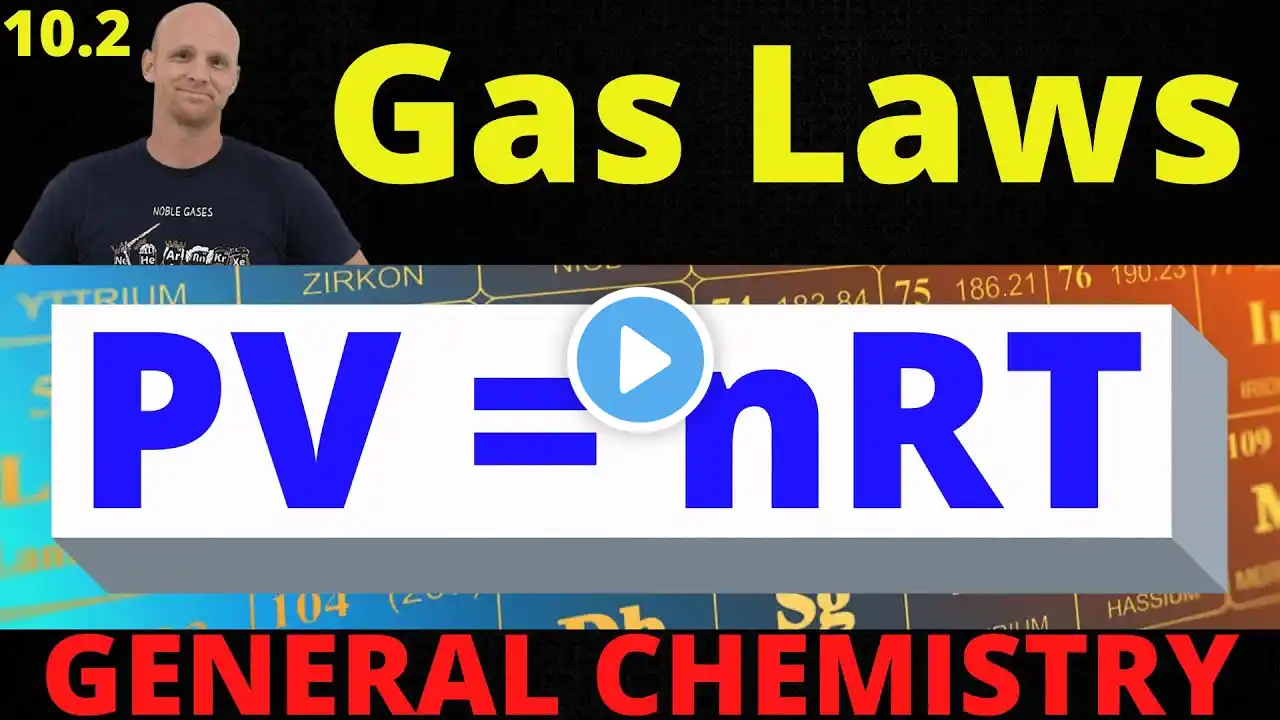
In this video, I review the five points of the Kinetic Molecular Theory (KMT), which essentially define what an ideal gas is. We look at the relationships between the variables in the ideal gas law (P, n, V, and T) and conceptual questions about these relationships. We look at Kinetic Energy and the equation KE = 1/2 mv2 and look at the variables that affect speed (temperature, or average kinetic energy, and the mass or molar mass). We also look at the Maxwell- Boltzmann distribution curve that shows how the velocity varies in a sample, and how a change in temperature or molar mass will shift the graph. Lastly, we look at rates of effusion/diffusion from both a conceptual standpoint and using Graham's equation. For an AP Chemistry class, according to the CollegeBoard AP Chemistry curriculum and course description, this mini lesson video would cover parts of Unit 3 Intermolecular Forces and Properties (3.4 Ideal Gas Law, 3.5 Kinetic Molecular Theory) #chemistry #apchemistry #chem #apchem #intermolecularforces #imfs #unit3 #tutorial #chemistrytutorial #chemhelp #chemistryhelp #APchemhelp #APchemistryhelp #minilesson #lesson #howto #review #chemistryreview #APchemistryreview #apchemistrytutorial #chemistryvideo #apchemistryvideo #flippedclassroom #gases #idealgas #idealgases #effusion #diffusion #Grahamslaw #maxwellboltzmann #molecularspeed #molecularvelocity #kineticenergy