General Chemistry Lecture 8 - Gases and Kinetic Molecular Theory
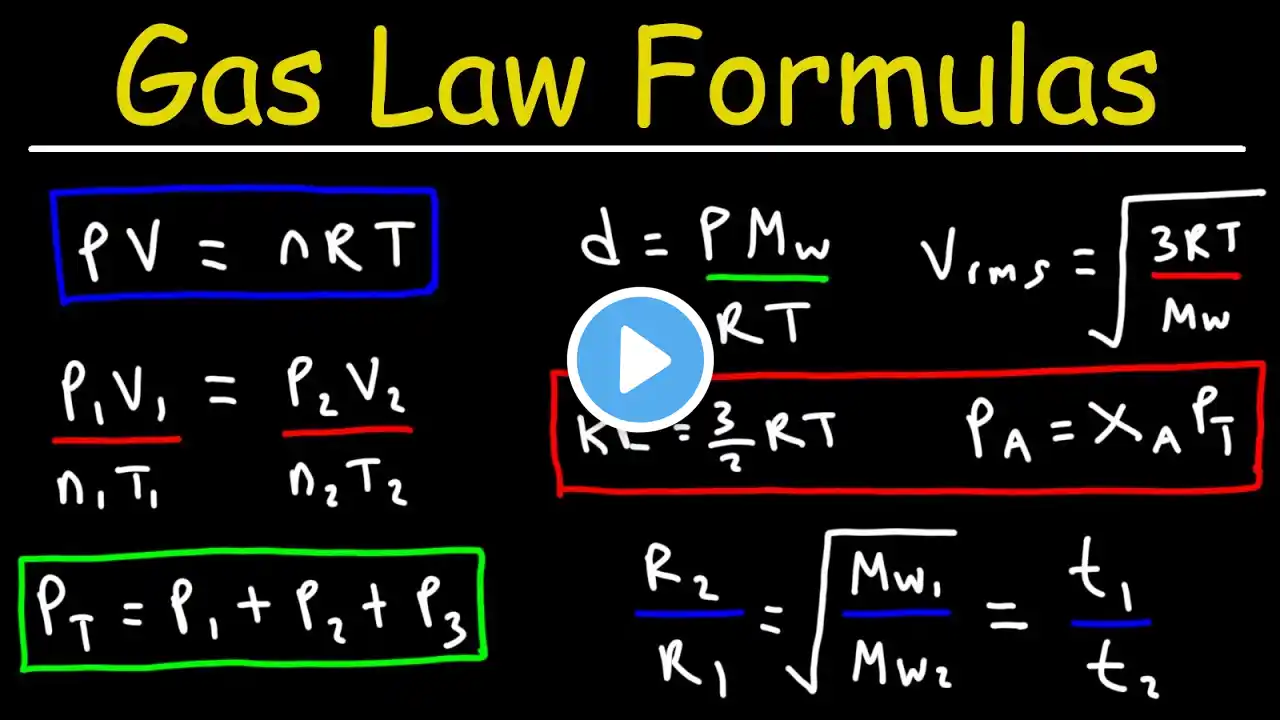
Full general chemistry lecture. Topics include explanation of gas laws, Dalton's Law of partial pressures, Kinetic molecular theory, effusion, diffusion, Graham's law of effusion, and finally ... van der Waal's gas equation! Video Thumbnail: Lots of gas phase chemistry in the photo from the scent of the mint tea vapors (at least I think its mint) and the diffusion of the eucalyptus oil candle (at least I think it is eucalyptus). Video Thumbnail Photo Credit: MS365 Powerpoint #apchem #apchemistry #chemistry #kineticmoleculartheory #temperature #kineticmoleculartheory #kinetictheoryofgases #kinetictheory #rootmeansquarevelocity #vanderwaalsequation #effusion #diffusion See playlist below for lecture videos • Gases – Laws, Behavior, and Molecular Motion Chapters include 00:00 Gases Introduced 00:15 Why gases exist 01:12 Pressure defined 01:42 Absolute zero and Kelvin explained 03:02 Boyle’s Law Explained 04:20 Boyle’s Law Example 05:49 Charles’s Law Explained 07:11 Charles’s Law Example 08:44 Avogadro’s Law Explained 10:01 Avogadro’s Law Example 11:31 Ideal Gas Law Explained 12:41 Ideal Gases Visualized 13:42 Ideal Gas Law Example 1: Finding Volume 15:13 Ideal Gas Law Example 2: Finding Moles 16.53 Combined Gas Law Explained 18:03 Combined Gas Law Example 20:37 What is STP in chemistry? 21:49 Finding the volume of one mole of ideal gas at STP 21:04 Density of ideal gases 24:11 Density of an Ideal Gas Example 1 25:31 Density of an Ideal Gas Example 2 27:03 Gas Stoichiometry Example 1 28:49 Gas Stoichiometry Example 2 30:56 Dalton’s Law of Partial Pressures Explained 31:55 Dalton’s Law of Partial Pressures Example 33:30 Gas Mole Fractions Explained 34:25 Using Mole Fractions to Find Partial Pressures Example 36:08 Kinetic Molecular Theory Explained 40:23 The Meaning of Temperature 46:02 Root Mean Square Velocity Explained and Example 48:47 effusion and diffusion explained 50:39 Graham’s Law of Effusion for Gases Explained 53:03 Graham’s Law of Effusion for Gases Example 54:54 Van der Waal’s Equation Explained 58:08 Van der Waal’s Equation Example