BEHAVIOR OF GASES GRADE 1O SCIENCE Quarter 4 Module 1. BOYLE'S LAW, CHARLES LAW, GAY-LUSSACS LAW.
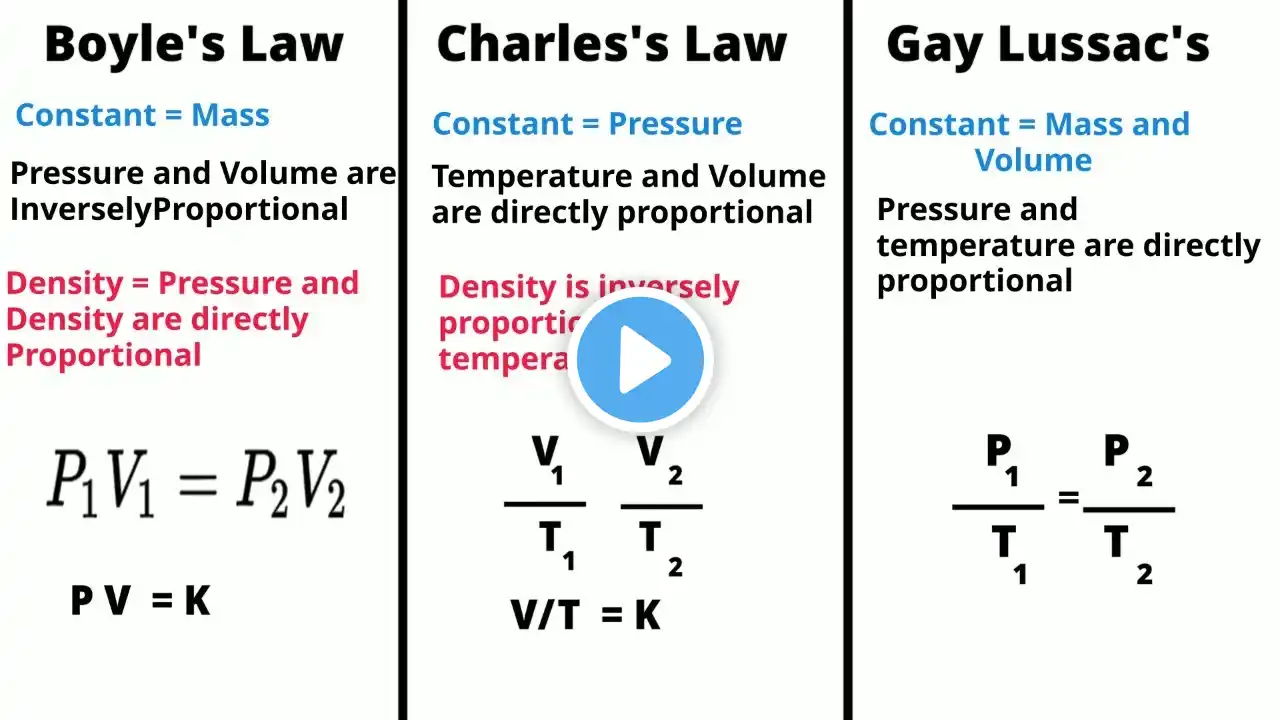
Download the powerpoint file here: https://www.raket.ph/janus_ada001 Download learning module here: https://drive.google.com/file/d/1rb0X... Most Essential Learning Competency: • Investigate the relationship between volume an pressure at constant temperature of a gas; volume and temperature at constant pressure of a gas; explains these relationships using kinetic molecular theory (S10MTIVa-b-21) Specifically, you should be able to: 1. Recognize the symbols used to describe gases such as n, P, R, T, V and STP; 2. Explain the interrelationships of pressure, volume and temperature 3. Solve problems involving changes in the condition of the gas using the equations for Boyle’s Law, Charles’ Law, Combined Gas Law, Gay-Lussac’s Law, Avogadro’s Law, Ideal Gas Law 4. Describe how gas density is related to pressure, temperature, and number of moles by the ideal gas law. 5. Determine the application of gas laws in daily occurrences. / @einsteinaticstv