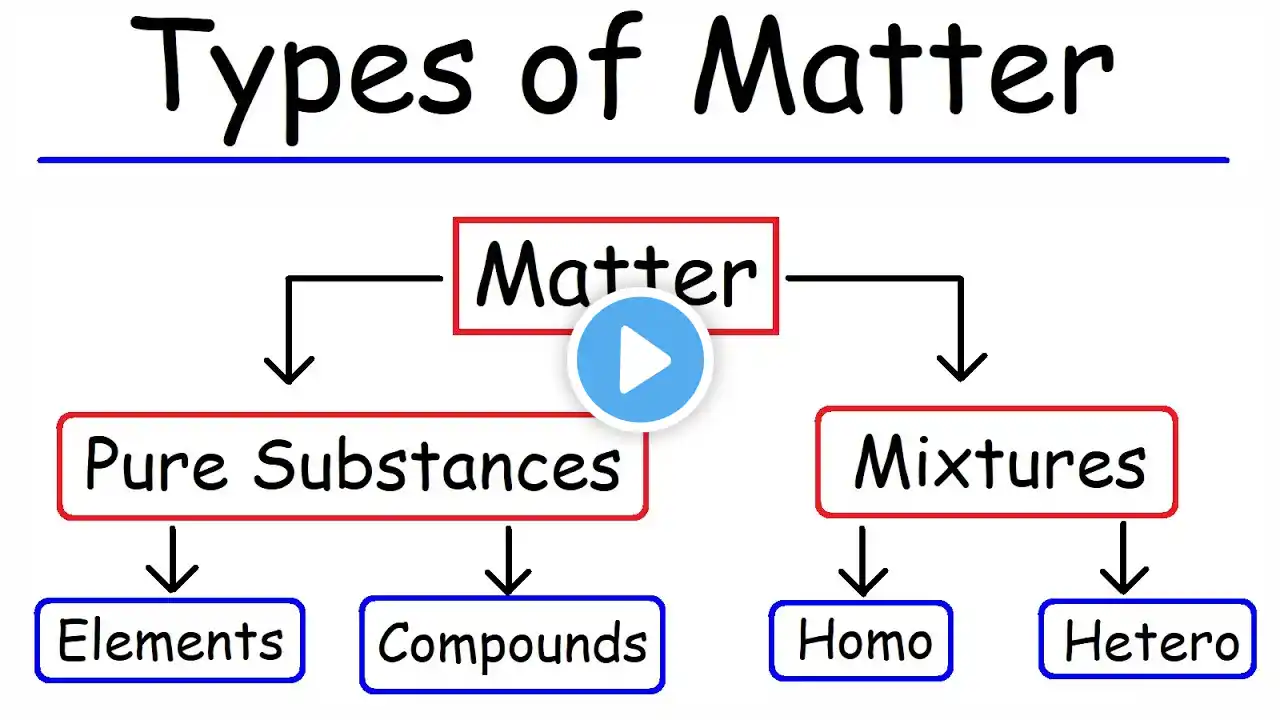
States of Matter, An introduction to different phase changes of matter and their examples.
Phase changes refer to the transitions that matter undergoes as it moves between different states, such as solid, liquid, and gas. These changes are driven by alterations in temperature and pressure. Here's an introduction to some common phase changes and their examples: Melting (Solid to Liquid): Melting occurs when a solid substance absorbs enough heat energy to break the bonds holding its particles in a fixed position, allowing them to move freely. Example: Ice melting into water when heated above 0°C. Freezing (Liquid to Solid): Freezing is the opposite of melting, where a liquid substance loses heat energy, causing its particles to slow down and form a solid structure. Example: Water freezing into ice when cooled below 0°C. Vaporization (Liquid to Gas): Vaporization involves the transformation of a liquid into a gas, either through evaporation or boiling. Evaporation occurs at the surface of a liquid, where molecules with sufficient kinetic energy escape into the surrounding space. Boiling occurs throughout the liquid when its vapor pressure equals the atmospheric pressure. Example: Boiling water to produce steam. Condensation (Gas to Liquid): Condensation is the process by which a gas transforms into a liquid when it loses heat energy. This can occur when gas molecules come into contact with a cooler surface or when the gas is cooled. Example: Water vapor in the air condensing into droplets to form clouds. Sublimation (Solid to Gas): Sublimation occurs when a solid transitions directly into a gas without passing through the liquid phase. This happens when the substance's vapor pressure exceeds atmospheric pressure. Example: Dry ice (solid carbon dioxide) sublimating into carbon dioxide gas. Deposition (Gas to Solid): Deposition is the reverse of sublimation, where a gas transforms directly into a solid. This usually occurs when gas molecules lose energy rapidly and condense onto a surface. Example: Frost forming on a cold surface as water vapor in the air deposits onto it. These phase changes are fundamental to understanding the behavior of matter under different conditions and are essential in various scientific and everyday contexts.