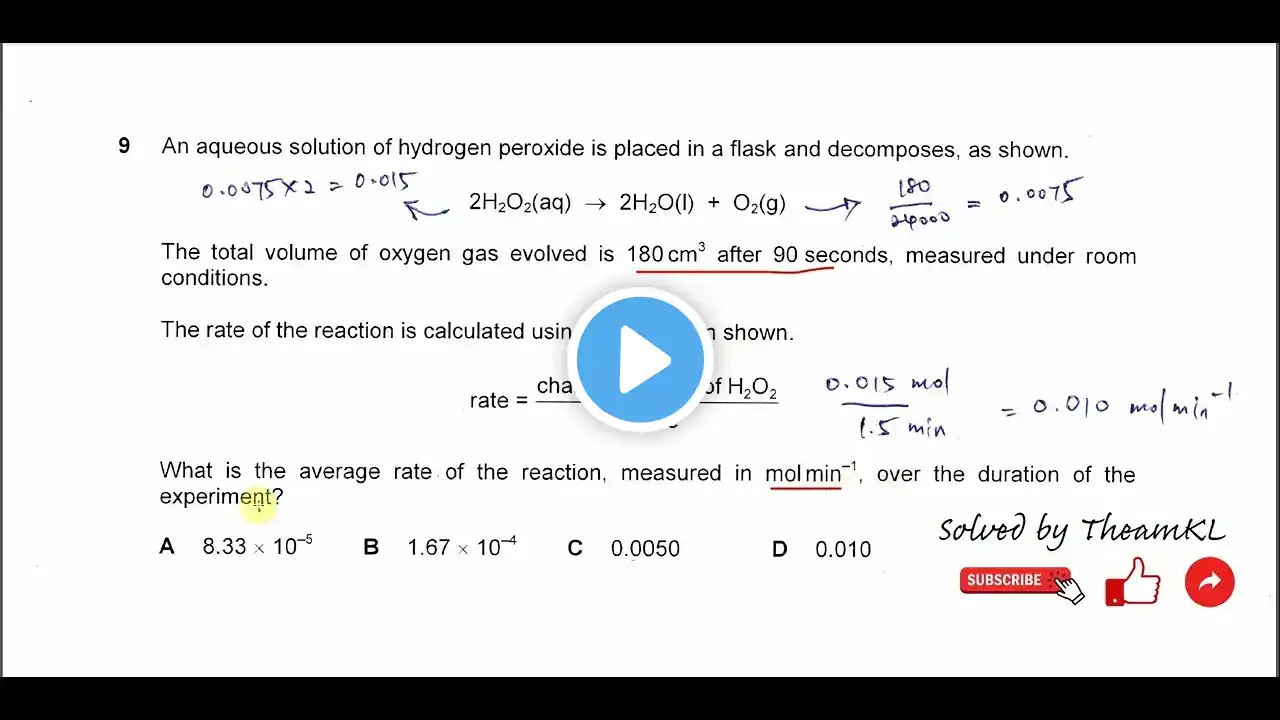
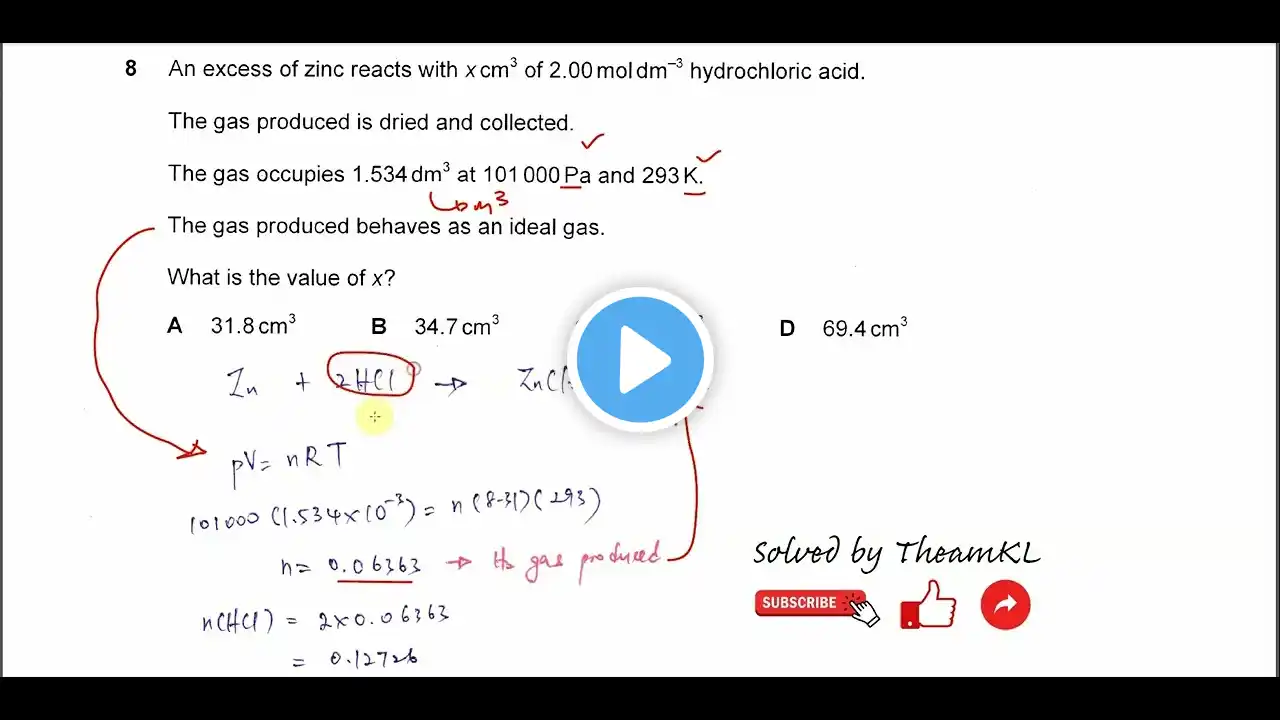
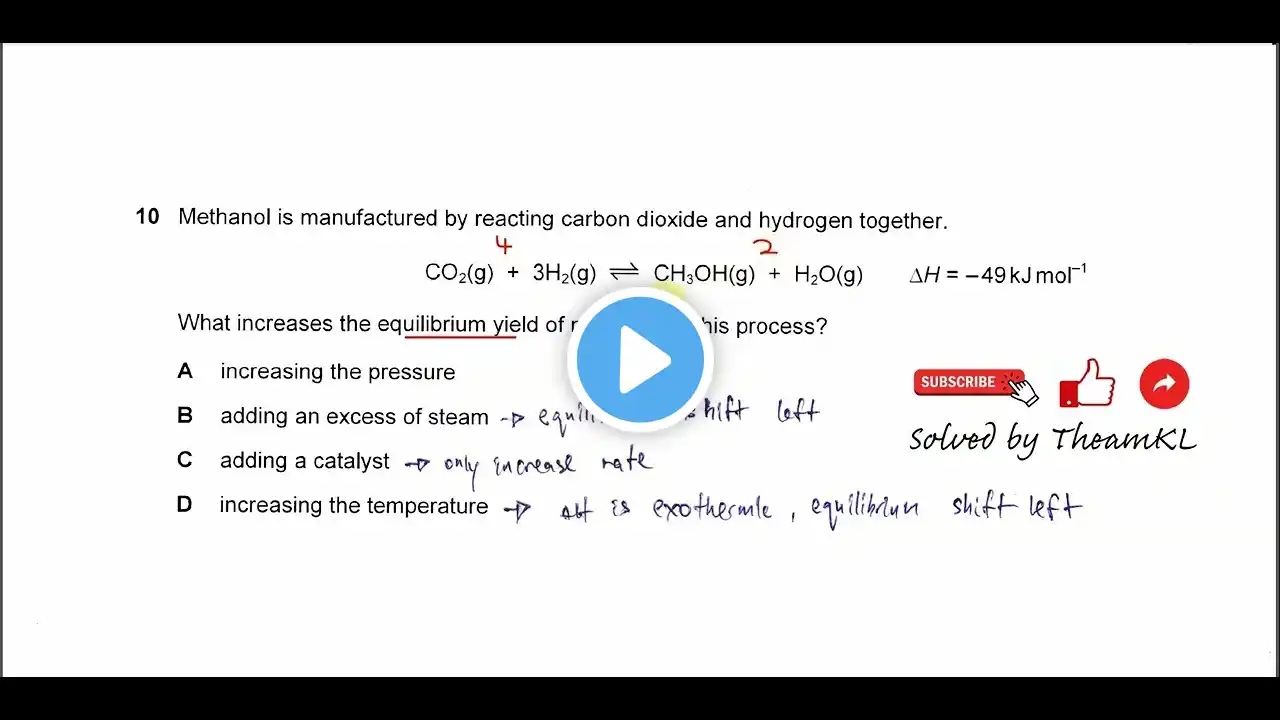
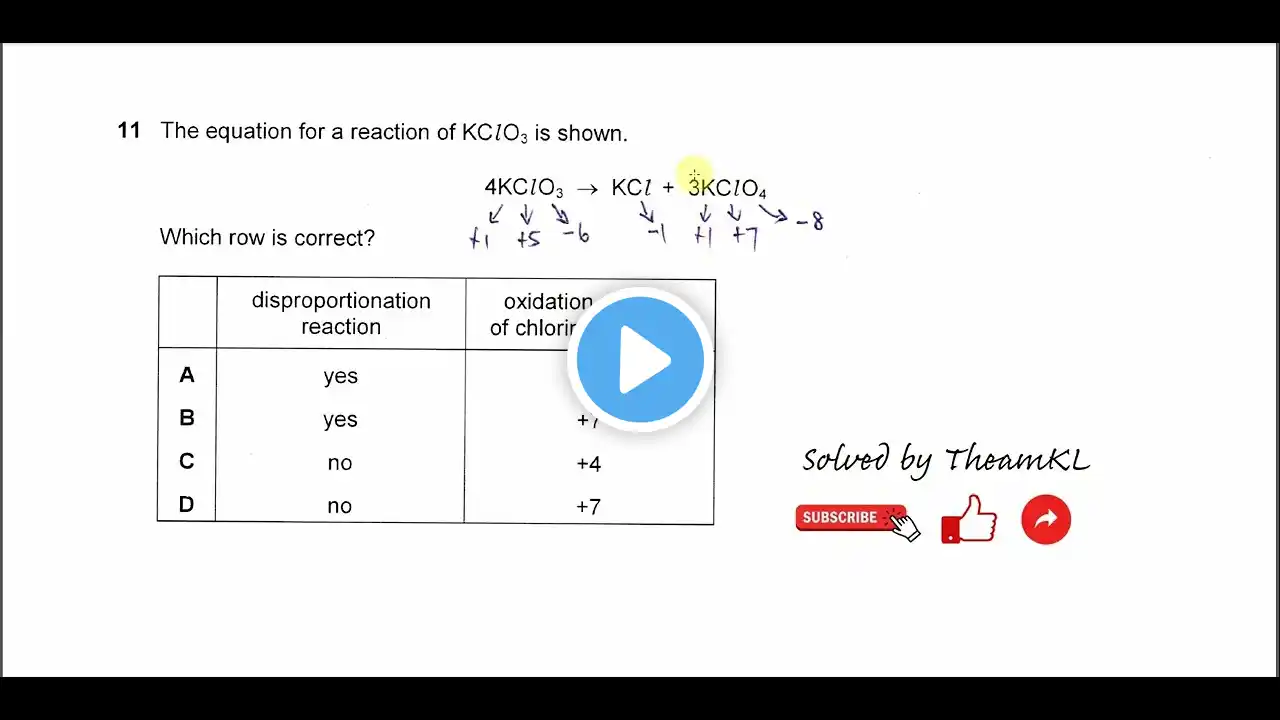
Chemical Equilibria | Chapter 8 – Cambridge International AS & A Level Chemistry
This chemistry chapter offers an extensive examination of chemical equilibria, beginning with the concept of a reversible reaction, defined as a chemical process where the products formed can react together to re-form the original reactants. The crucial state discussed is dynamic equilibrium, which is achieved when the rate of the forward reaction becomes exactly equal to the rate of the backward (or reverse) reaction, resulting in the concentrations of both reactants and products remaining constant. Establishing this state requires a closed system, where no matter, such as gaseous products, can escape from the reaction mixture. The position of equilibrium, which reflects the relative amounts of products versus reactants, can be disturbed and shifted according to Le Chatelier’s principle, a foundational rule stating that if a stress is applied to a system at equilibrium, the position will move in a direction that minimizes that change. Applying this principle allows qualitative deduction of the effects of changing concentration, pressure, and temperature: increasing a reactant concentration shifts the position to the right (towards products); increasing pressure shifts the position toward the side of the equation containing the fewer total moles of gas molecules; and increasing temperature favors the endothermic reaction (the direction that absorbs energy), while decreasing temperature favors the exothermic reaction (the direction that releases energy). However, the presence of a catalyst increases the rates of both forward and reverse reactions equally, reducing the time needed to reach equilibrium but having no effect on the final position. The chapter also develops the quantitative concepts of the equilibrium constant in terms of concentration (K c ) and the equilibrium constant in terms of partial pressures (K p ), allowing for equilibrium calculations using concentrations or gas partial pressures, the latter of which are calculated using mole fractions. A critical distinction is made regarding equilibrium constant values: only a change in temperature will alter the numerical value of K c or K p ; changes in pressure or concentration only shift the position of equilibrium. These equilibrium principles are essential for industrial processes, such as the Haber process for synthesizing ammonia and the Contact process for manufacturing sulfuric acid, where conditions are chosen to maximize yield and efficiency based on Le Chatelier’s principle. Finally, the chapter transitions to acid-base equilibria, introducing the Brønsted–Lowry theory, which defines acids as proton donors and bases as proton acceptors. Acids and bases are further classified as strong (fully dissociated in aqueous solution, e.g., hydrochloric acid) or weak (partially dissociated, e.g., ethanoic acid or aqueous ammonia). This difference in dissociation determines the solution’s pH and allows differentiation between strong and weak acids based on qualitative tests like electrical conductivity and reactivity with reactive metals. The chapter concludes by explaining the use of indicators—weak acids or bases with different conjugate colors—and how their selection for acid-base titrations depends on matching their specific pH color range to the steep portion of the relevant titration curve (e.g., strong acid-strong base, strong acid-weak base). 📘 Read full blog summaries for every chapter: https://lastminutelecture.com 📘 Have a book recommendation? Submit your suggestion here: https://forms.gle/y7vQQ6WHoNgKeJmh8 Thank you for being a part of our little Last Minute Lecture family! ⚠️ Disclaimer: These summaries are created for educational and entertainment purposes only. They provide transformative commentary and paraphrased overviews to help students understand key ideas from the referenced textbooks. Last Minute Lecture is not affiliated with, sponsored by, or endorsed by any textbook publisher or author. All textbook titles, names, and cover images—when shown—are used under nominative fair use solely for identification of the work being discussed. Some portions of the writing and narration are generated with AI-assisted tools to enhance accessibility and consistency. While every effort has been made to ensure accuracy, these materials are intended to supplement—not replace—official course readings, lectures, or professional study resources. Always refer to the original textbook and instructor guidance for complete and authoritative information.