Thermodynamics Lecture 5 | Work Done, Sign Convention & Mathematical Derivation | JEE-NEET
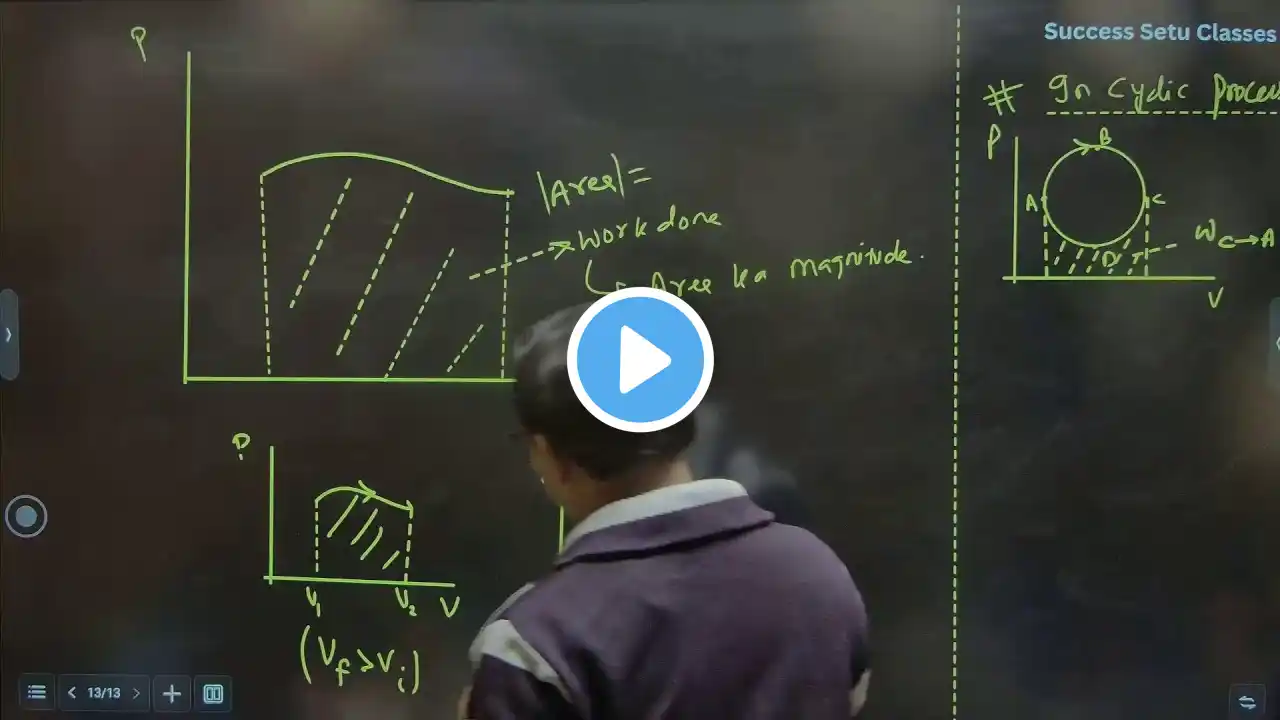
Thermodynamics Lecture 5 | Work Done, Sign Convention & Mathematical Derivation | Class 11 NEET | ChembyKKSir ⸻ #Thermodynamics #Thermochemistry #ThermodynamicsLecture5 #PhysicalChemistry #Class11Chemistry #NEETChemistry #NCERTChemistry #WorkDone #PVWork #SignConvention #IsothermalProcess #IsochoricProcess #FreeExpansion #ReversibleProcess #IrreversibleProcess #FirstLawOfThermodynamics #HeatAndWork #InternalEnergy #ChemistryByKKSir #ChembyKKSir #NEET2025 ⸻ Namaste Dear Students 🙏 Welcome to Thermodynamics & Thermochemistry — Lecture 5, one of the most important, conceptual and scoring lectures of Thermodynamics, where the entire concept of WORK DONE is completed with full theory, derivation, sign convention and process-wise discussion, strictly as per NCERT Class 11 and NEET syllabus. This lecture is a direct continuation of Lecture 3 and Lecture 4, where we covered thermodynamic systems, state and path functions, reversible–irreversible processes and thermodynamic equilibrium. In this lecture, those concepts are applied mathematically and logically to understand how work is defined, calculated and interpreted in thermodynamic systems. In this lecture, I — Krishna Kumar Sah (ChembyKKSir) — have explained from the very basics what work is, why work is a path function, how work depends on the process, the sign convention of work, and the complete mathematical derivation of work done step by step. Topics covered in this lecture include: • Meaning and definition of work in thermodynamics • Difference between mechanical work and thermodynamic work • Work as a path function • Role of external pressure in work calculation • Infinitesimal work done • Complete derivation of work expression • δw = −P(external) dV • Integral form of work • w = − ∫ P(external) dV • Physical meaning of negative sign in work Work done in different thermodynamic processes explained in detail: • Free expansion process – External pressure equal to zero – Why work done is zero • Isochoric process (constant volume) – ΔV = 0 – Why work done is zero • Isothermal reversible process – Meaning of isothermal process – Conditions for reversibility – Step-by-step derivation of work done – Importance of reversible work in thermodynamics Sign convention of work (very important for NEET): • Work done by the system • Work done on the system • Expansion work and compression work • Common sign mistakes made by students Progress of Thermodynamics & Thermochemistry till this lecture: • Introduction to Thermodynamics • Introduction to Thermochemistry • Importance and scope of Thermodynamics • System, Surroundings and Boundary • Open, Closed and Isolated Systems • Thermodynamic parameters • State function and Path function • Reversible and Irreversible processes • Thermodynamic equilibrium (Thermal, Mechanical and Chemical) • Concept of work • Mathematical expression of work • Work in different thermodynamic processes This lecture builds a strong foundation for Heat, Internal Energy and the First Law of Thermodynamics. If the concept of work is clear, thermodynamics becomes logical, numerical-friendly and highly scoring for NEET. ⸻ Instagram: / chembykksir WhatsApp Channel: https://whatsapp.com/channel/0029Vb6F... Facebook: https://www.facebook.com/share/1ACSwY... LinkedIn: / krishna-kumar-sah-13708661 Telegram: https://t.me/chembykksir YouTube: / @chembykksir ⸻ If work is understood with sign and logic, the First Law becomes easy and fearless. Revise patiently, practice numericals and trust the process.