Why Is Work Done Zero In An Isochoric Process? - Thermodynamics For Everyone
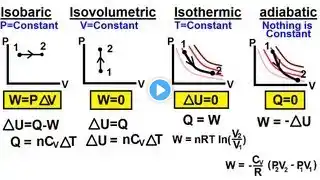
Why Is Work Done Zero In An Isochoric Process? Have you ever wondered why certain processes in thermodynamics involve no work being done? In this informative video, we'll explain the reasons behind the absence of work in an isochoric process. We'll start by defining what an isochoric process is and what makes it unique compared to other thermodynamic processes. We'll discuss how energy transfer occurs during heating or cooling when the volume remains constant. You'll learn how the concept of work relates to changes in volume and why, in an isochoric process, the work done is always zero despite fluctuations in pressure and temperature. We'll also explore practical examples, such as how engines and heating systems operate during steps where volume stays fixed. Additionally, we'll show you how to calculate work in thermodynamic systems and why the volume change is essential for work to be performed. Whether you're a student, educator, or enthusiast, understanding this principle is key to grasping many real-world applications in energy transfer and engine cycles. Join us for this clear and straightforward explanation, and subscribe to our channel for more accessible insights into thermodynamics and energy principles. ⬇️ Subscribe to our channel for more valuable insights. 🔗Subscribe: https://www.youtube.com/@Thermodynami... #Thermodynamics #Physics #EnergyTransfer #WorkInThermodynamics #IsochoricProcess #HeatTransfer #EngineCycles #PhysicsEducation #ScienceExplained #EnergyPrinciples #ScienceForEveryone #PhysicsTutorial #ThermodynamicsBasics #EnergySystems #PhysicsMadeSimple About Us: Welcome to Thermodynamics For Everyone! Our channel is dedicated to making the principles of thermodynamics accessible to everyone. We cover essential topics such as the laws of thermodynamics, heat transfer, entropy, energy systems, thermal efficiency, and much more. Whether you're curious about heat engines, the Carnot cycle, or the behavior of gases, our content is designed to help you grasp these concepts in an engaging way.