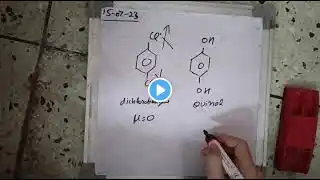
Dipole Moment of Quinol & Dichlorobenzene | JEE/NEET Chemistry | Pathankot class12
NEET & JEE Chemistry Concept – Dipole Moment Confused why quinol (hydroquinone) has a non-zero dipole moment? What about ortho, meta, and para-dichlorobenzene — which one is polar and why? This video gives a crystal-clear explanation with logic, diagrams, and competitive exam focus. 🔍 Topics Covered: – Dipole moment of quinol (benzene-1,4-diol) – Comparison of o-, m-, and p-dichlorobenzene – Concept of vector addition of dipoles – Application in NEET, JEE Mains, and Class 12 Boards 📍 Special focus for Pathankot students 💻 Also useful for All India aspirants preparing for JEE/NEET 🧪 Learn chemistry deeply, with real-life logic and visualization. 📲 Join offline/online classes in Pathankot for NEET/JEE chemistry. DipoleMoment #Quinol #Dichlorobenzene #NEETChemistry #JEEChemistry #ChemistryShorts #PathankotStudents #NEET2025 #JEE2025 #Class12Chemistry #ElectromericEffect #OrganicChemistry #PathankotCoaching #AllIndiaStudents