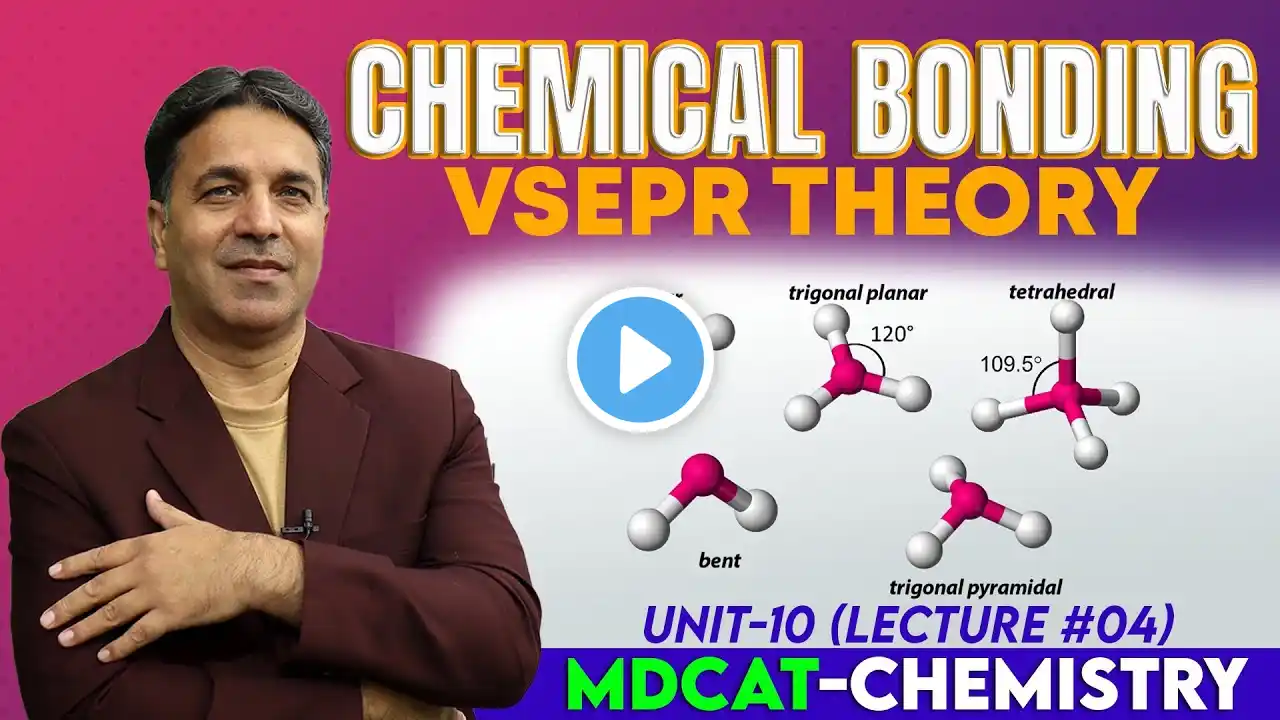
Molecular Orbital Theory (MOT) Postulates | Chapter 3 Lec 10 | 11th Class Chemistry | New Book 2025
Welcome to ChemBooster! This is Lecture 10 of Chapter 3 – Chemical Bonding, from the 11th Class Chemistry – New Syllabus 2025 (Punjab Board). In this lecture, we cover: ✅ Concept of Molecular Orbital Theory (MOT) ✅ Postulates and types of Molecular Orbitals ✅ Types of overlaps: s–s overlap and p–p overlap ✅ Molecular Orbital Diagram of the Hydrogen Molecule (H₂) This lecture will help you clearly understand how atomic orbitals combine to form molecular orbitals and how the bond formation in molecules can be explained using the Molecular Orbital Theory. 📌 Subscribe to ChemBooster 🔔 for complete 11th Class Chemistry coverage, topic-wise Shorts, and smart concept breakdowns as per the New Curriculum 2025. #ChemBooster #11thClassChemistry #newbook2025 #Chapter3 #ChemicalBonding #MolecularOrbitalTheory #MOT #MolecularOrbitals #HydrogenMolecule #NewSyllabus2025 #newsyllabus2025 #newbook #newcurriculum2025 #ChemistryLecture #ScienceEducation #LearnChemistry #ChemistryMadeEasy #IGCSEChemistry #ScienceEducation #LearnChemistry #LearnPhysics #LearnBiology #HighSchoolScience #STEMEducation #ScienceStudents #ChemistryMadeEasy #PhysicsMadeEasy #BiologyMadeEasy #StudyWithMe #ExamPreparation #BoardExams #SchoolLife #EducationForAll #SmartLearning #StudyTips #ScienceTeacher #YouTubeLearning #GlobalEducation #Highschool #Highschoolchemistry #IGCSE #OLevelChemistry #OLevelPhysics #OLevelBiology #AlevelChemistry #AlevelPhysics #AlevelBiology