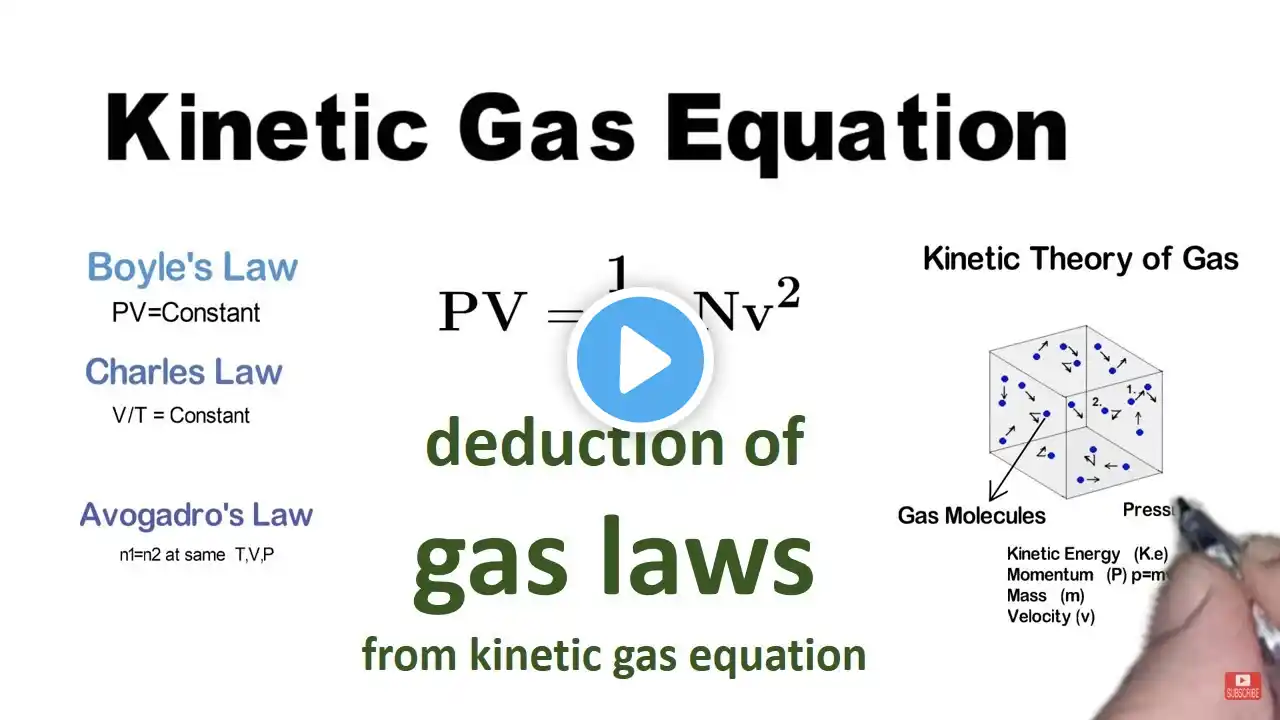
Deriving Boyle's Law, Charles' Law, and Avogadro's Law from the Kinetic Gas Equation
deduction of gas laws from kinetic gas equation The kinetic theory of gases explains the behavior of gases based on the motion of individual molecules. Using this theory, we can derive three important gas laws: Boyle's Law, Charles' Law, and Avogadro's Law, which describe the relationships between pressure, volume, temperature, and the number of gas molecules. The Kinetic Gas Equation The kinetic gas equation relates pressure, volume, the number of gas molecules, and their motion. It shows that pressure is proportional to the number of molecules and their speed, and inversely proportional to the volume. Derivation of Boyle's Law Boyle's Law states that, at constant temperature, the pressure of a gas is inversely proportional to its volume. When temperature is constant, an increase in volume results in a decrease in pressure, because the molecules have more space and collide with the walls of the container less frequently. Derivation of Charles' Law Charles' Law says that, at constant pressure, the volume of a gas is directly proportional to its temperature. As the temperature rises, the molecules move faster, causing the gas to expand and increase in volume to maintain constant pressure. Derivation of Avogadro's Law Avogadro’s Law states that the volume of a gas is directly proportional to the number of molecules when the temperature and pressure are constant. Increasing the number of gas molecules requires the volume to increase, so the pressure remains constant. Conclusion These gas laws can be derived from the kinetic gas equation, showing how the microscopic behavior of gas molecules influences macroscopic properties like pressure, volume, and temperature. #KineticGasEquation #BoylesLaw #CharlesLaw #AvogadrosLaw #GasLaws #KineticTheory #IdealGas #Physics #Chemistry #Pressure #Volume #Temperature



















