🔥 Thermodynamics Formula Sheet Made Easy | All Basic Formulas for NEET JEE 😍⚡ Chemistry concept
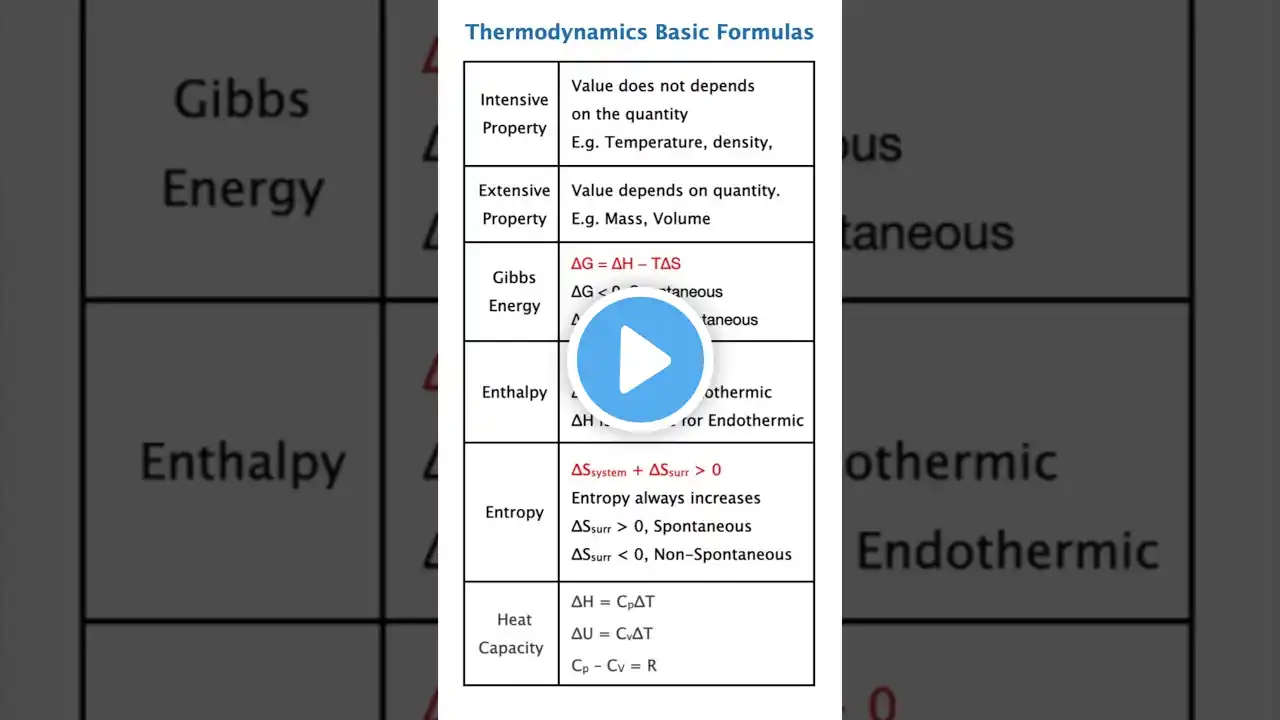
Introduction – Thermodynamics Without Fear 😍 Hello toppers! 👋 Thermodynamics becomes scoring when you understand formulas, not memorise them. In this video, we simplify all basic thermodynamics formulas using daily-life logic, smiley learning 😄, and exam shortcuts. This video is useful for: Chemistry for IIT JEE, NEET, Class 11, Class 12, GATE, CSIR NET, UGC NET, TIFR, CUTE, SET. What Is Thermodynamics? 📘 Thermodynamics studies the relationship between: ✔️ Heat ✔️ Work ✔️ Energy It explains how energy moves and transforms. 💬 Memory Trick: Thermo means heat, dynamics means change 😄 Most Important Formula – First Law 🌟 Change in internal energy equals heat plus work. ✔️ Heat added increases internal energy ✔️ Work done by system decreases internal energy 💬 Shortcut: Energy change equals energy in minus energy out. Work Done Formula Simplified 🧠 Work equals pressure multiplied by change in volume. ✔️ Expansion gives positive work ✔️ Compression gives negative work 💬 Exam Tip: Work equals area under PV curve. Internal Energy Made Easy 😄 Internal energy is total energy stored inside a system. ✔️ Depends only on temperature ✔️ Independent of path 💬 Analogy: Internal energy is like money in your wallet 💰 Important Process Formulas 📝 Isothermal Change in internal energy equals zero Heat equals work Isochoric Work equals zero Heat equals change in internal energy Isobaric Work equals pressure times change in volume Adiabatic Heat equals zero Change in internal energy equals negative work 💬 One Line Trick: First find what is zero 😄 Entropy and Spontaneity Basics 🚀 Entropy measures randomness. ✔️ Higher entropy means more disorder ✔️ Natural processes increase entropy 💬 Memory Trick: Messy room equals high entropy 😄 Gibbs Free Energy Formula ⚡ Delta G equals delta H minus T δ S. ✔️ Delta G negative means spontaneous ✔️ Delta G positive means non spontaneous 💬 Exam Shortcut: Spontaneous reactions have negative Gibbs energy. Quick Notes, Exam Shortcuts, Must-Know Points 🧾 ✔️ First law connects heat, work, energy ✔️ Work is path dependent ✔️ Internal energy is state function ✔️ Isothermal internal energy change zero ✔️ Adiabatic heat zero ✔️ Gibbs energy predicts spontaneity 💬 Rank Booster Tip: Formula based questions give sure marks 😍 Summary & Motivation 🌈 Excellent work topper! 🎉 You now understand thermodynamics basic formulas with logic and clarity. You learned: ✔️ First law formula ✔️ Work formulas ✔️ Process shortcuts ✔️ Entropy basics ✔️ Gibbs energy formula Perfect for NEET, JEE, Class 11–12, GATE, CSIR NET, UGC NET, SET, TIFR, CUTE. Keep learning smart — thermodynamics becomes fun when formulas make sense 😄 🔥 Like, Share, Subscribe for more formula-based chemistry hacks! 🔥 #Thermodynamics #ThermodynamicsFormulas #NEET #JEE #IITJEE #NEETChemistry #ChemistryFormulas #StudySmart #Class11Chemistry #Class12Chemistry #CSIRNET #GATEChemistry #UGCNET #TIFRExam #SETExam #CUTEExam #HeatWorkEnergy #FirstLawThermodynamics #GibbsEnergy #Entropy #ChemistryShorts #NEET2026 #JEE2026 #ScienceIsFun #ExamTips #FormulaRevision #ThermoBasics #ChemistryMadeEasy #RankBooster #ChemistryMotivation