Hess Law Class 11 | Hess Law of Constant Heat Summation in Urdu
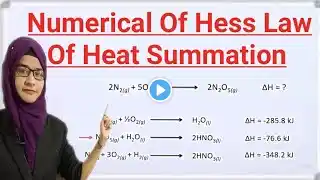
Hess Law Class 11 | Hess Law of Constant Heat Summation in Urdu Welcome to our video on Hess Law of Constant Heat Summation, where we will explain how this fundamental concept in chemistry works and its importance in calculating the heat of reaction. Hess Law states that the total enthalpy change for a chemical reaction is the same regardless of the number of steps taken to reach the final products. In this video, we will break down the concept of Hess Law and show you how to apply it to solve problems in thermochemistry. Understanding Hess Law is crucial for predicting and understanding the energy changes that occur during chemical reactions. So sit back, relax, and let us guide you through the world of constant heat summation with Hess Law. Don't forget to like, comment, and subscribe for more educational content on chemistry and science! Stay tuned for a clear explanation of Hess Law and its applications in real-world scenarios. Let's dive in!