Rutherford Discovery of the Atomic Nucleus
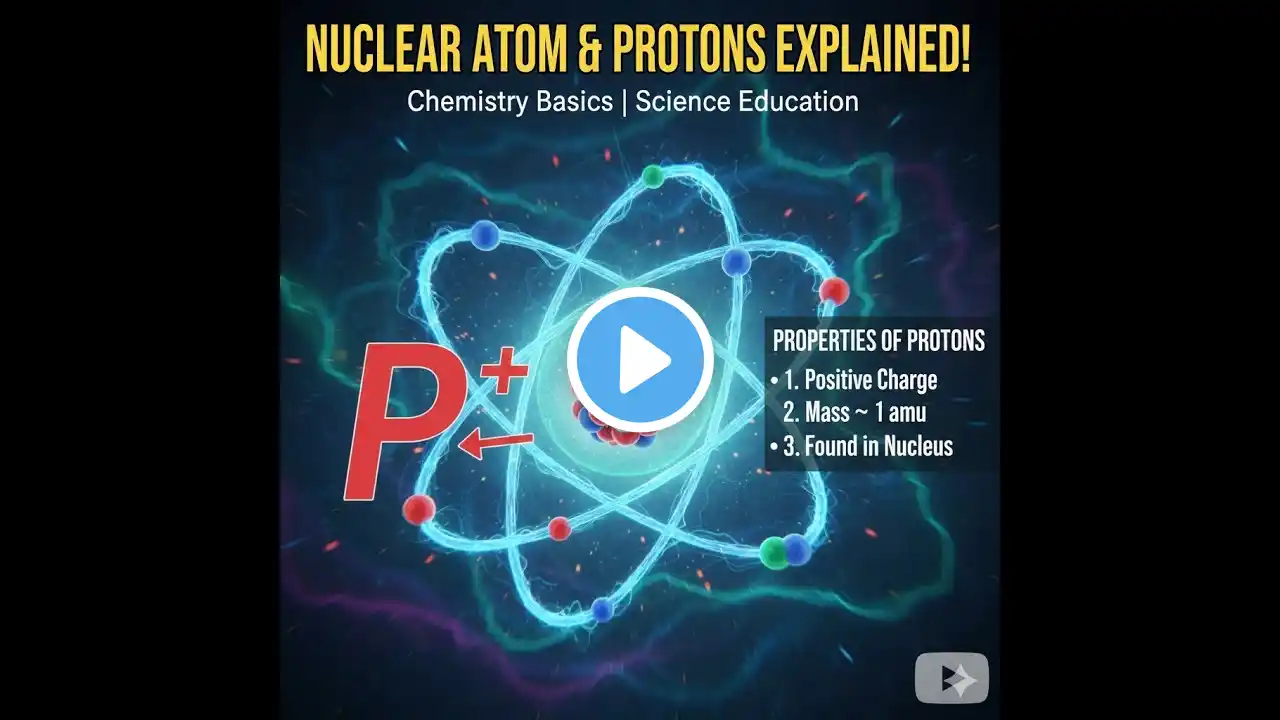
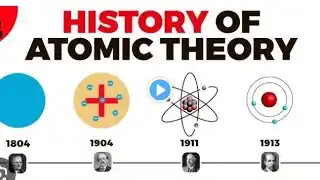
This video details the `discovery of nucleus` by Ernest Rutherford, walking through his scientific workflow. It explains the `rutherford scattering experiment`, including the `gold foil experiment` where `alpha particle` deflection revealed the dense core of the atom. This pivotal work fundamentally changed our understanding of `atomic structure`. Explore how Ernest Rutherford’s gold-foil experiment revealed the atomic nucleus by measuring α-particle scattering. Learn how this elegant 1911 discovery transformed atomic theory and launched the era of modern nuclear physics. Rutherford’s gold foil experiment is the moment atomic theory became testable physics. This video shows how Geiger and Marsden fired fast alpha particles at an ultra-thin gold foil expecting only small deflections (as in Thomson’s “plum pudding” atom), but instead found a shocking result: while most particles passed straight through, a tiny fraction bounced back at large angles. The only explanation was that almost all positive charge and mass are packed into a tiny, dense nucleus, with electrons occupying the vast empty space around it. By treating the interaction as Coulomb repulsion from a compact charge, Rutherford’s scattering law matched the angular data and turned “the atom” from a vague sphere into a measurable structure — launching modern nuclear physics and setting the stage for Bohr’s quantized orbits and, later, quantum mechanics. What you will learn: Why the plum-pudding model predicted only gentle scattering How the gold foil setup detected single-particle deflections What large-angle backscattering implies about charge concentration The core idea behind Rutherford scattering (angle ↔ closest approach) Why atoms are mostly empty space, and how big the nucleus is How this experiment opened the path to Bohr’s model and nuclear physics Timestamps: 00:00 – Why test the atom at all? 01:11 – Gold foil setup: alpha source, foil, scintillation screen 02:21 – The surprise: rare but huge deflections 03:31 – Coulomb scattering and the nuclear hypothesis 04:46 – The new atom: tiny nucleus, vast empty space 07:11 – Deducing nuclear size and charge scaling with Z 08:26 – Bohr’s refinement and early validation 09:46 – From scattering to nuclear physics and transmutation 11:01 – Detection ingenuity: counting flashes by eye 14:46 – Conclusion: “gold foil revealed the atom’s heart” #Rutherford #GoldFoilExperiment #AtomicNucleus #NuclearPhysics #AtomicStructure #AlphaScattering #BohrModel