States of Matter | Chemistry Class 9 | Chapter 1 | New Book, 2025
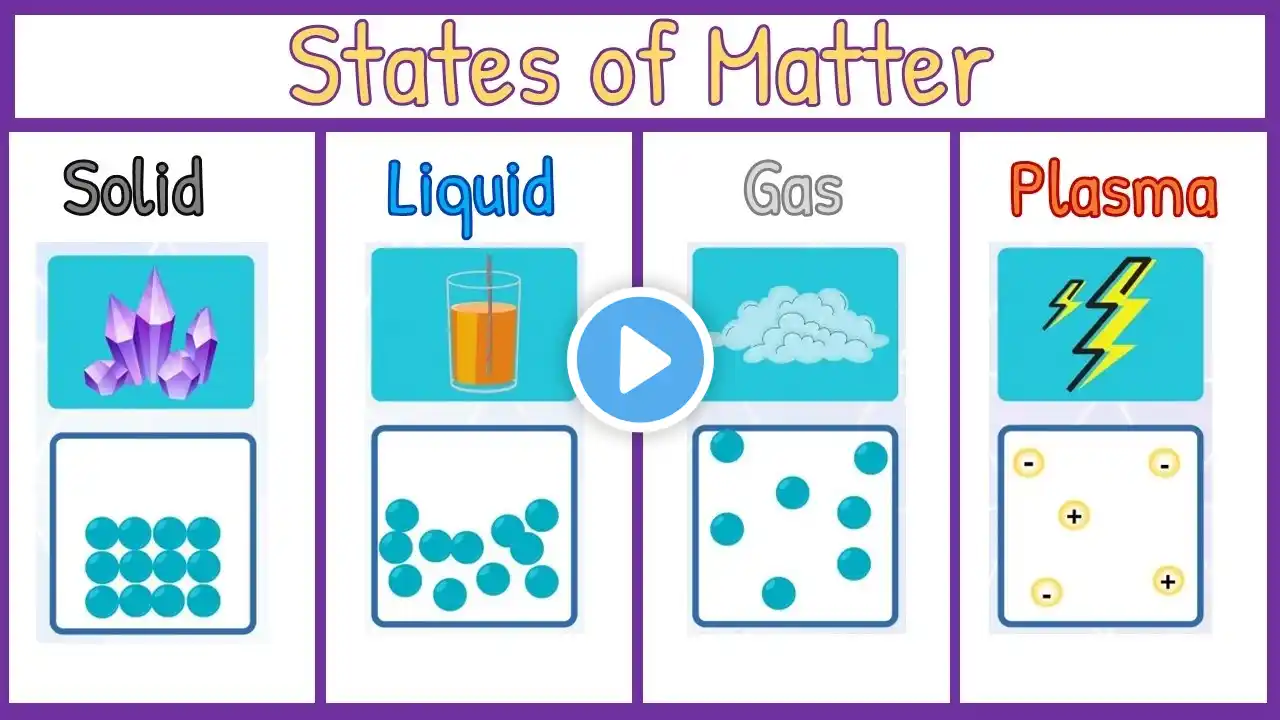
Welcome to Learn with Ease..! States of Matter | Chemistry Class 9 | Chapter 1 | New Book, 2025 Welcome to this detailed lecture on States of Matter from Class 9 Chemistry, Chapter 1 of the New Book 2025. In this video, we will explore one of the most fundamental concepts in chemistry: the different states in which matter can exist. 🌍 Introduction to States of Matter Matter is anything that has mass and occupies space. Depending on the atomic structure and molecular arrangement, matter exists in different forms. These forms are called the states of matter. Traditionally, we study three main states: solids, liquids, and gases, but modern science has introduced us to additional states like plasma, supercritical fluids, liquid crystals, graphene, and other exotic states of matter. 🧱 Solids Solids are characterized by strong intermolecular forces, fixed molecular arrangement** in either crystalline solids or amorphous solids, high density, rigidity and low compressibility. Examples include metals, salts, and diamonds. 💧 Liquids Liquids have weaker intermolecular forces than solids, definite volume but no definite shape, fluidity allowing them to flow, moderate density, and slight compressibility. Liquids also undergo important phase transitions such as melting point, boiling point, condensation, and evaporation. 🌬️ Gases Gases have very weak intermolecular forces, no fixed shape or volume, high compressibility, low density, and the ability to expand and fill any container. ⚡ Plasma Plasma is called the fourth state of matter. It forms when gases are ionized at high temperature** or pressure. Plasma contains charged particles and shows high conductivity. Examples include lightning, stars, and fluorescent lamps. Plasma is vital in physics and astronomy. 🧪 Supercritical Fluids 🔬 Liquid Crystals Liquid crystals have properties between liquids and solids. Molecules show order like solids but still have fluidity like liquids. They are used in display technology such as LCD screens. 🧩 Graphene Graphene is an exotic state of matter consisting of a single layer of carbon atoms in a hexagonal pattern. It shows extraordinary conductivity, strength, and flexibility, making it crucial in modern materials science. 🌌 Exotic States of Matter Other exotic states like Bose-Einstein condensates and fermionic condensates exist under extreme temperature and pressure. These states help scientists understand quantum-level behavior of matter. 📚 Importance for Class 9 Students This chapter is the foundation of chemistry, linking to advanced topics in physics, materials science, and engineering. By learning about solids, liquids, gases, plasma, supercritical fluids, liquid crystals, graphene, and exotic states, students will better understand matter at microscopic and macroscopic levels. In the New Book 2025, this is Chapter 1, showing its importance for all later topics. 📌 Conclusion The study of states of matter is the starting point of chemistry. It explains how matter behaves under different conditions of temperature and pressure, and why materials show different properties. From solids, liquids, and gases to advanced forms like plasma, graphene, and exotic states, this chapter opens the door to understanding matter deeply. This video makes Class 9 Chemistry Chapter 1 (New Book 2025) easy, engaging, and exam-focused. Watch till the end, take notes, and revise regularly. ✨ Don’t forget to like, share, and subscribe for more Class 9 Chemistry New Book 2025 lectures. Comment below for questions or clarifications. #StatesOfMatter #Class9Chemistry #NewBook2025 #Solids #Liquids #Gases #Plasma #Graphene #LiquidCrystals #PhaseDiagram