What are Covalent Bonds? Types of Covalent Bonds | Chemical Bonding
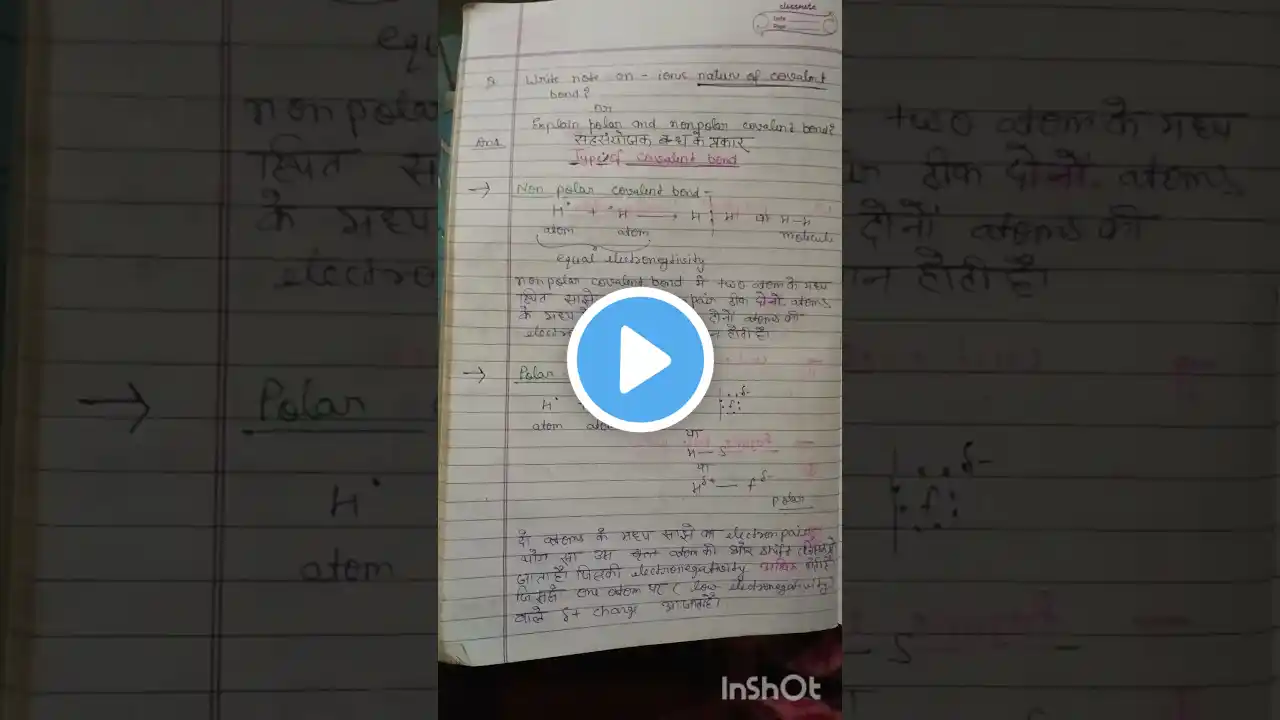
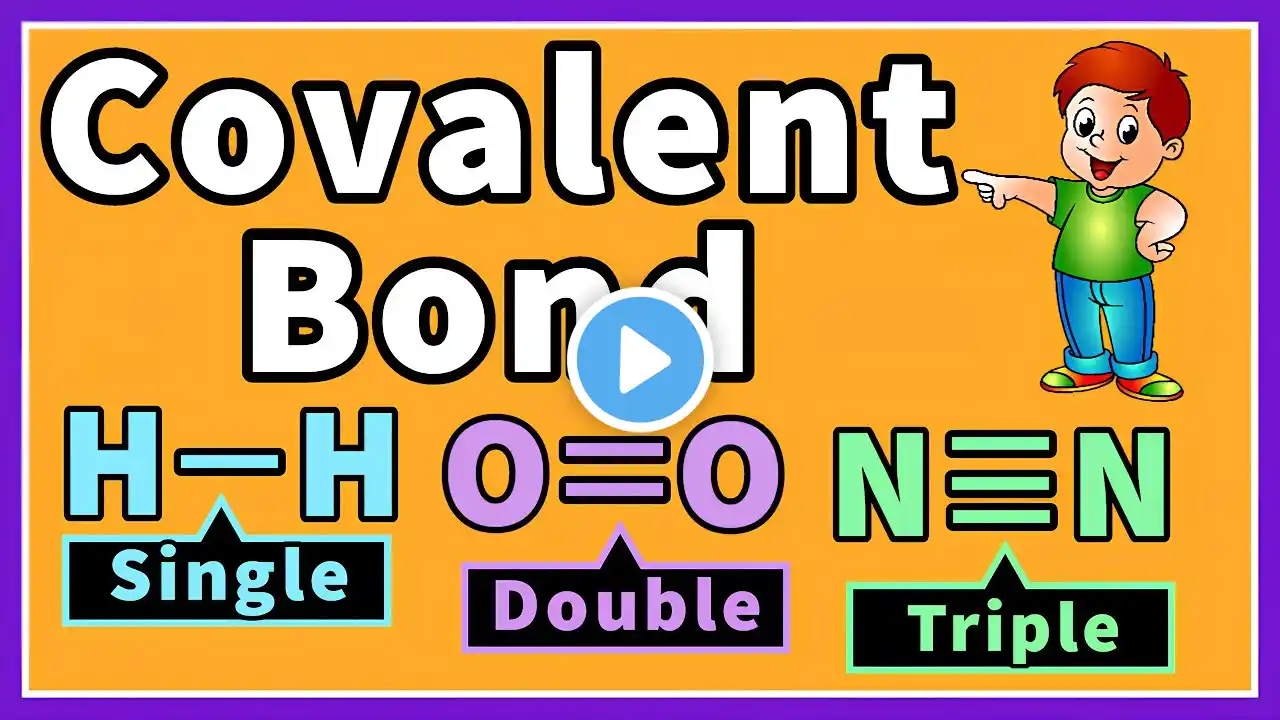
What is a covalent bond? A covalent bond is a chemical bond, that is formed by mutual sharing of electrons, between two non-metals. Let's take the example of chlorine. which is a non-metal. and has seven electrons in its, outermost shell. In order to achieve stable electronic configuration. it needs one more electron. because according to the octet rule, an atom becomes stable by attaining 8 electrons in their Valence shell. if another chlorine atom comes along, it also needs one more electron, to complete its outermost shell. How do both atoms attain, 8 electrons? they achieved stable electronic configuration, by sharing their outermost electrons. This sharing of electrons creates a bond, known as Covalent bond. There are 3 types of Covalent Bonds. Single Covalent Bond Double Covalent Bond Triple Covalent Bond #covalentbond #covalent #covalentbonding #covalentbonds #chemicalbond #bond #chemistry #education #science For more informative videos please subscribe to our channel. @afzaalchemist