#topic-2/Gaseous State/ Types of graphs on Charle's law/ #chemistry #chemgateacademy #education#iit
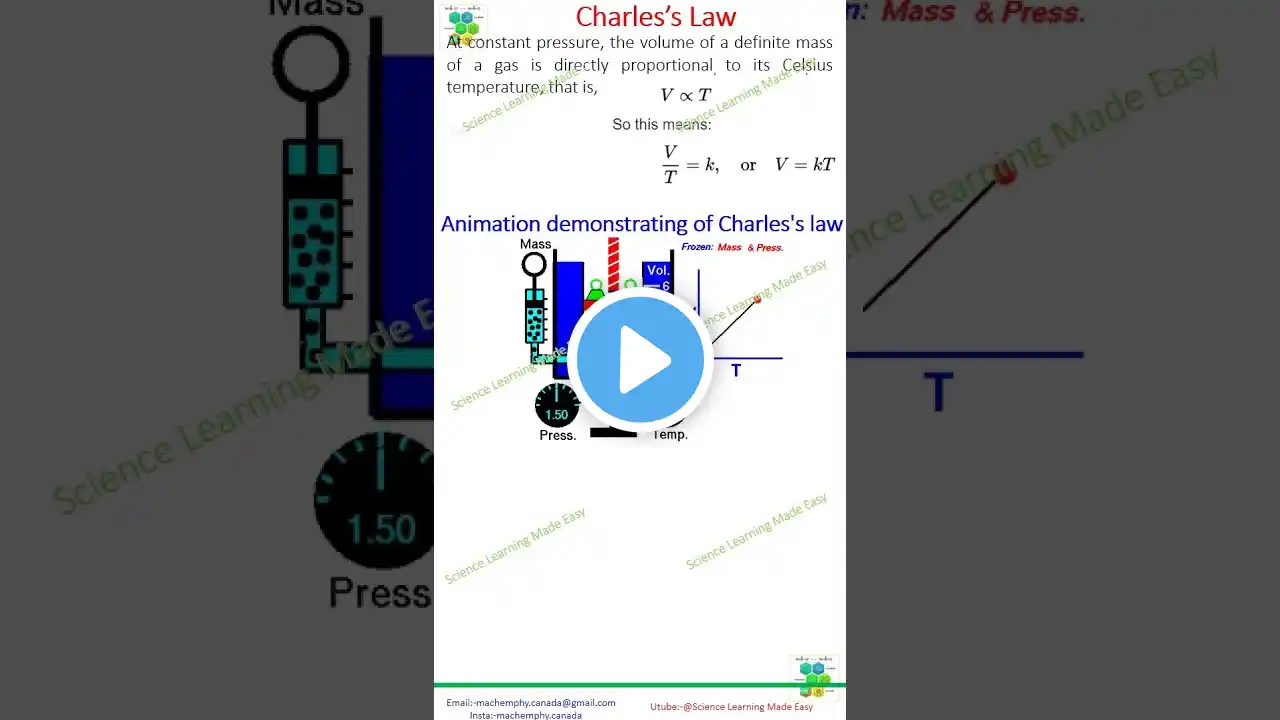
The gaseous state is a fascinating area of study in chemistry, and one of the fundamental principles governing this state is Charles's Law. Charles's Law describes how gases expand when heated, stating that the volume of a given mass of gas is directly proportional to its temperature, provided the pressure remains constant. As the temperature of a gas increases, so does its volume; conversely, as the temperature decreases, the volume contracts. This relationship is critical in understanding the behavior of gases under various thermal conditions. To visualize Charles's Law, we often use graphical representations that make the concept clearer and more accessible. A common graph used to illustrate Charles's Law is a plot of volume (V) against temperature (T) for a fixed amount of gas at constant pressure. When this graph is plotted, it results in a straight line, demonstrating the direct proportionality between volume and temperature. The line typically extends from a certain point on the temperature axis (often extrapolated to absolute zero in Kelvin), highlighting how volume theoretically approaches zero as the temperature decreases. Another useful graph for Charles's Law is a plot of volume versus temperature on different scales, such as Celsius or Kelvin. When using the Kelvin scale, the graph is particularly straightforward and linear, emphasizing the direct relationship. However, when using the Celsius scale, the graph needs careful interpretation, especially when extrapolating to extremely low temperatures, where the volume might approach zero. Charles's Law has significant applications in various scientific and industrial contexts, such as understanding the behavior of hot air balloons, predicting the expansion of gases in engines, and explaining natural phenomena like the behavior of gases in the atmosphere. By studying the graphical representations of Charles's Law, students and professionals gain a deeper understanding of how gases behave under different temperatures, which is essential in many areas of science and engineering. #chemistryrocks #charleslaw #gaslaws #scienceisawesome #chemistrymagic #stemlearning #scienceexploration #learnwithfun #PhysicsAndChemistry #ChemistryClassroom #chemistryforall #scienceeducation #chemistrycommunity #scienceinaction #chemistryconcepts #chemistrysimplified #sciencefacts #educationalcontent #scienceandmath #chemistryteachers