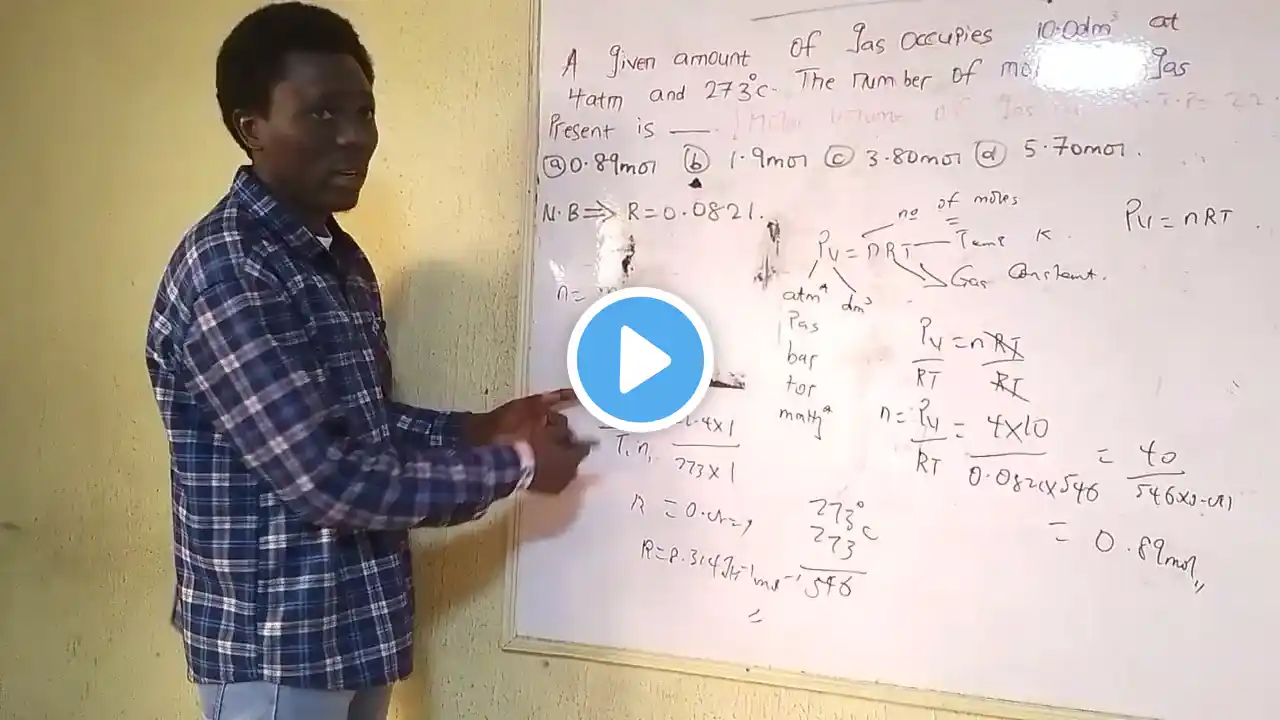
IDEAL GAS EQUATION (pv=nRT)
There are four quantities to put in consideration when Calculations involving gases are given. 1. Volume 2.Pressure 3. Temperature and 4.Number of Moles. Three of these parameters appear in the general gas equation; But for ideal gas equation, pv=nRT where p is in pressure in ATM,V is volume in dm³,T is temperature in Kelvin and n is number of moles. Remember the value for R to be 0.0821 atm dm³K–¹ mol–¹. Let me also add this; Dalton's law of partial pressure (Applicable to Gases mainly) it State that if there is a mixture of gases which do not react chemically together,then the total pressure exerted by the mixture is the sum of the partial pressure of the individual gases that make up the mixture.